All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Creative Biolabs provides versatile T cell receptor (TCR) clinical diagnostics service platform. Based on our multiply techniques such as Southern blot, PCR and flow cytometry, we can detect TCR gene rearrangement to identify T cell proliferations in T-cell caused blood cancer, immunodeficiency, autoimmune disease, transplant rejection and tolerance, and infectious disease.
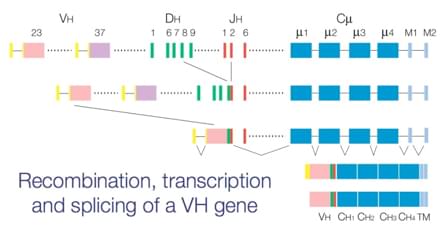
TCR molecules are responsible for explicitly recognizing foreign from self-peptides bound to major histocompatibility complex (MHC) molecules, resulting in T–cell activation and clearance of antigen. As a kind of membrane bound heterodimers, an individual T cell receptor is composed of two polypeptide chains (αβ or γδ) linked by a disulfide bond and associated with a non-polymorphic cytoplasmic membrane bound complex of proteins collectively known as CD3. Each of these TCR chains is encoded by distinct DNA elements known as variable (V), joining (J), diversity (D), and constant (C) gene segments. TCR gene rearrangement is thought to occur in T cell ontogeny, which plays an important role in antigen recognition by T lymphocytes and enables T cells to recognize antigens specifically, and any dysregulation in this complex yet highly regulated process may lead to disease.
Since the dysregulation of TCR gene rearrangement can cause certain kinds of diseases and the traditional diagnoses of lymphoma and leukemia such as histological methods usually have limitations making tumor cells undetectable in blood or bone marrow samples. Creative Biolabs has integrated a plenty of advanced molecular biological techniques, including southern blot hybridisation, polymerase chain reaction and flow cytometry to offer a TCR diagnostics platform for the detection of the TCR rearrangement and for the characterization of T cell proliferations in malignancy and in diseases. What's more, Creative Biolabs is professional in next generation sequence (NGS) field and we can also provide NGS-based TCR analysis to help customers study disease pathogenesis at a genomic level, which has been proven to has the potential to expand diagnostic accuracy, improve monitoring of disease recurrence, and enhance minimal residual disease testing. We are confident about that our molecular level analysis of TCR gene rearrangement can offer a great help in clinical diagnosis of various tumors and other diseases related to TCR expansions involved pathogenesis.
Creative Biolabs has mature TCR diagnostic platform which can fit your specific needs in clinical trials or other studies. We strive to retain our customers through strong communication, timeliness and responsive TCR clinical diagnostics program.
Reference:
Diagnostic role of tests for T cell receptor (TCR) genes.
To discuss your demands or to request a proposal, please contact us by .
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION