Creative Biolabs antibody cysteine modification platform has the unprecedented ability to produce antibodies introduced free cysteine without disrupting any antigen-targeting capacity, stability or homogeneity, which cannot be otherwise produced in any other platform. Advantages include quality, stability, purity, rapidly delivery as well as reduced production and development cost. This service has a wide application including but not limited to molecular imaging, antibody-drug conjugations (ADCs) and diagnostics.
Modification of proteins by the covalent attachment of payloads has gained prominence in the development of therapeutic proteins. In the past decade, many novel site-specific, bio-orthogonal reactions that enable precise and controlled modification of proteins have been reported. Comparing with the primary amines of lysines, it takes more advantages to choose sulfhydryls of cysteines serve as conjugation sites. Because this amino acid is less abundant and forms conserved disulfide bridges in antibodies to eliminate the risk of leading to steric hindrance of target recognition, we use both conventional modification methods and novel precise modification at engineered C- or N-terminal free cysteines of antibodies, such as site-specific mutagenesis and traceless cleavable linkers.
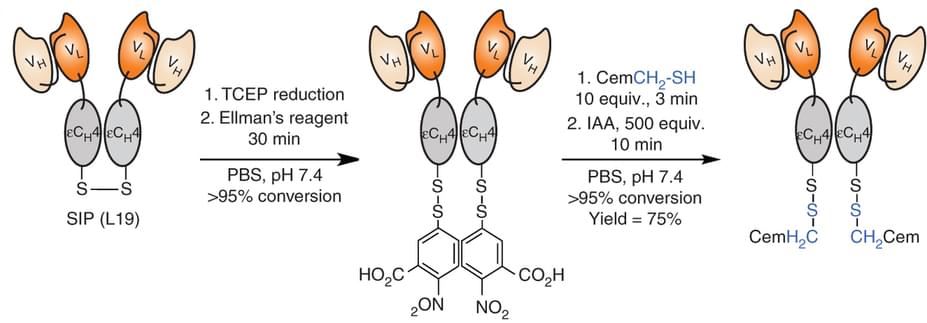 Fig. 1 Construction
of a traceless chemically defined tumor-vascular targeting antibody-drug
conjugate based on disulfide linkage. (Bernardes et al. 2013)
Fig. 1 Construction
of a traceless chemically defined tumor-vascular targeting antibody-drug
conjugate based on disulfide linkage. (Bernardes et al. 2013)
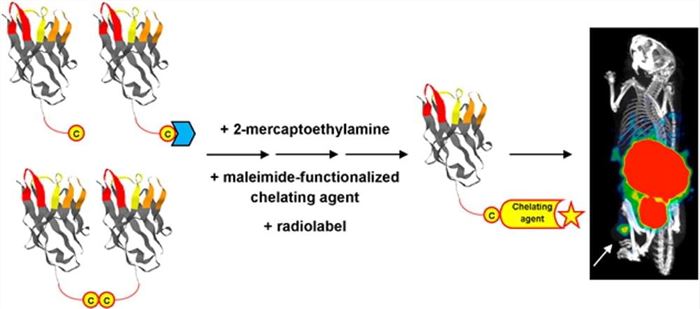 Fig. 2 Massa et al. (2014)
Fig. 2 Massa et al. (2014)
Guarantees
We guarantee any antibody cysteine modification (over 95% purity) with excellent homogeneity and antigen-binding properties.
Competitive advantages
Expertise knowledge and rich experience in site-specific modification and conjugation;
A comprehensive system of both conventional and modern novel methods and technologies for biomodification and conjugation;
Professional computational modelling and computer analysis design for site-specific modification residues selection.
Quality Controls Measures
The following quality control measures are employed to create the highest quality monoclonal antibodies commercially available: ELISA against the antigen, HPLC.
References
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.