Peptide Linker
With years of experience in recombinant antibody production, antibody engineering, bio-conjugation, and with the addition of the advanced “DrugLnk” organic synthesis platform, Creative Biolabs has estabilished a leading role in antibody-drug onjugates (ADCs) developments. We offer high-quality customized services for the design and synthesis of peptide linker and drug-peptide linker complexes to facilitate the development of next-generation ADCs with enahnced plasma stability.
Peptide linkers belong to protease-sensitivity linkers (also called enzymatically cleavable linkers) that have gained significant attention in ADC development due to their superior plasma stability and controled payload release mechanism. The core structure of a peptide linker comprises of either a di-peptide or a tetra-peptide that is recognized and cleaved by lysosomal enzymes to ensure the intracelluar drug release once the ADC is internalized and trafficked to the lysozome. Based on the core, additional components are added to the short peptide to facilitate antibody conjugation (e.g. Maleimide or PEG-Maleimide) or drug release (self-immolative moieties such as para-amino benzyl alcohol) in its most “native” form, another feature that makes peptide linkers appealing choices for ADC applications.
Comparing to chemically cleavable linkers (pH-sensitive linkers or disulfide linkers) peptide linkers exert enhanced serum stability. The scission of peptidic bonds in a peptide linker relies on lysosomal proteolytic enzymes that have very low activities in blood criculation. Early peptides linkers used in ADCs developments include tetra-peptides such as Gly-Phe-Leu-Gly and Ala-Leu-Ala-Leu. These first generation peptide linkers showed limitations in relatively slow drug release and a tendency for aggregation upon payload coupling. These issues have been circumvented in recent years with the development of new-generation dipeptide linkers such as Val-Cit and Phe-Lys linkers. These optimized small peptide linkers are cleaved by lysosomal extracts and purified human cathepsin B and they have been successfully applied in a few innovated ADCs. For instance, the Val-Cit (vc) linker has been utilized in several ADCs developed using auristatin-based payloads, such as MMAE or MMAF. By introducing self-immolative spacers, these highly potent, totally synthetic microtubule inhibitors are released inside the tumor cell with minimum distortion of their endogenous structures. Besides auristatin, other ADC payloads such as duocarmycin, mitomycin C, camptothecin, tallysomycin… have all been conjugated antibodies using small dipeptide linkers. To this point, several ADCs containing peptide linkers, including brentuximab vedotin, glembatumumab vedotin, MDX-1203, SGN-75, PSMA-ADC… are under extensive evaluation in clinical trials.
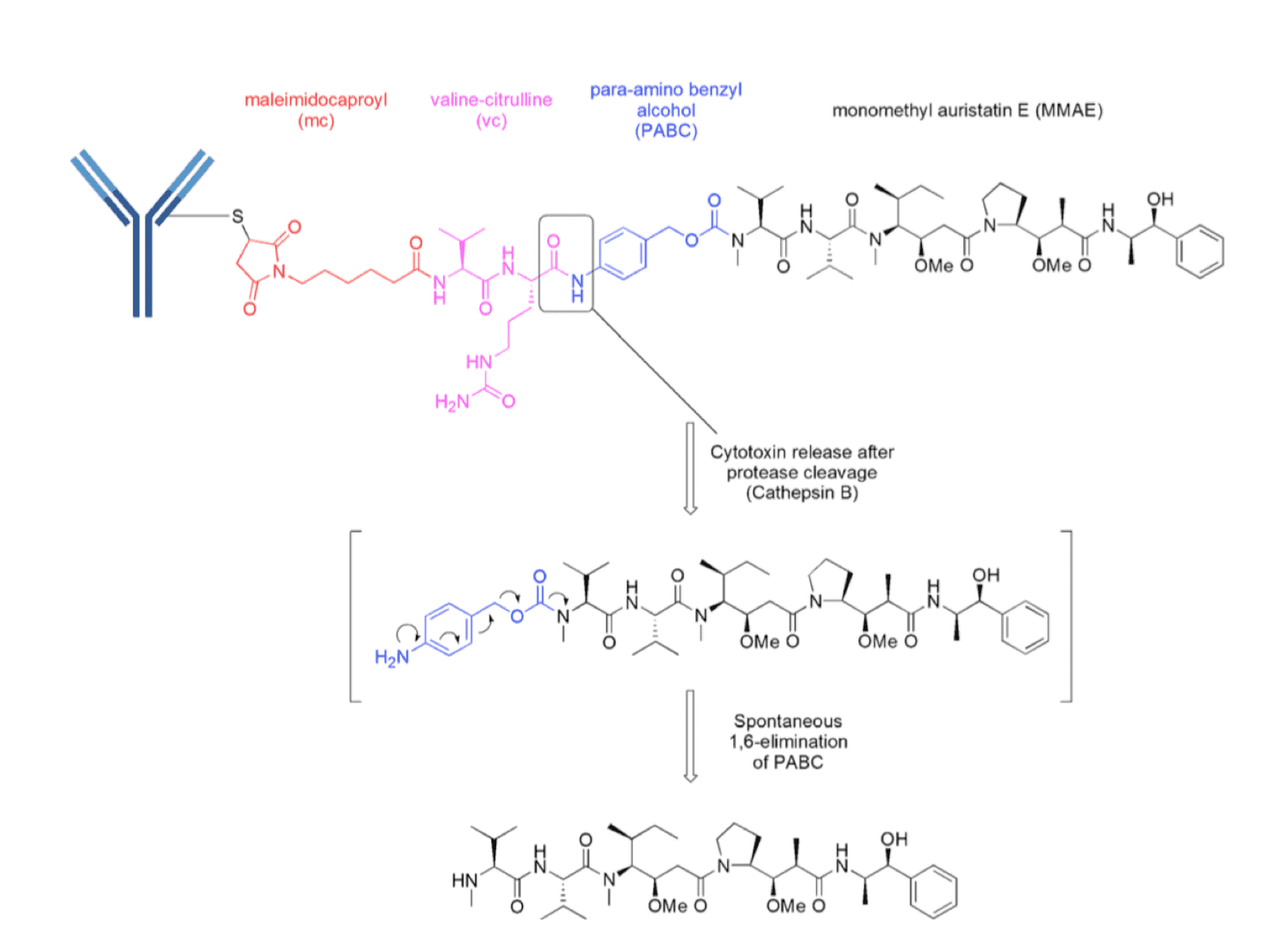 Schematic representation of an ADC with a enzymatically cleavable vc dipeptide linker. MMAE is attached to a protease recognition sequence valine-citrulline (vc) via a para-amino benzyl alcohol self-immolative moiety to ensure the release of MMAE in its “native” form (Pharm. Res., 2015).
Schematic representation of an ADC with a enzymatically cleavable vc dipeptide linker. MMAE is attached to a protease recognition sequence valine-citrulline (vc) via a para-amino benzyl alcohol self-immolative moiety to ensure the release of MMAE in its “native” form (Pharm. Res., 2015).
Scientists at Creative Biolabs are experinced with peptide linker and drug-peptide linker complex preparation, including Mc-vc-PAB-MMAE, Mc-vc-PAB-MMAF, Mc-va-PBD dimer, Mc-vc-PAB-CM-seco-DUBA…. To fulfill customers’ specific demands, with the advanced “DrugLnk” platform, we are dedicated in providing customized design services using peptide linkers for ADC developments. Depending on the antibody, payload drug, and tumor target, we will design or select the most suited linker to achieve targeted drug delivery. In the meantime, we also provide other services for the benefit of ADC development. Please feel free to contact us for more information and a detailed quote.
References:
- Wu, A.M.; Senter, P.D. Arming antibodies: prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005, 23(9): 1137-1146.
- Nolting, B.; et al. Linker technologies for antibody-drug conjugates. Methods. Mol. Biol. 2013, 1045: 71-100.
- Jain, N.; et al. Current ADC linker chemistry. Pharm. Res. 2015, 32: 3526-3540.
- McCombs, J.R.; Owen, S.C. Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J. 2015, 17(2): 339-351.
For Research Use Only. NOT FOR CLINICAL USE.
Related Sections
DrugLnk™ Custom Synthesis: One-stop ADC Development Service:
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
Contact usUSA
Tel:
Fax:
Email:
Europe
Tel:
Email:
Germany
Tel:
Email:

