One of the most important fundamental technologies in antibody production is hybridoma technology, development of which opens up the field of therapeutic antibodies development. Hybridoma technology firstly be described by Köhler and Milstein in 1975, was the groundbreaking advancement that led to monoclonal antibodies based modern antibody therapeutics. As noted in a biographical dictionary of Nobel Prize winners, a substantial commercial trade based on the industrial exploitation of hybridoma technology had begun by the early 1980s, thus turning monoclonal antibodies into one of the “success stories” not only of modern biomedical research, but also of commercial biotechnology.
Hybridoma was initially developed as a tool for studying the immune system as it allowed production of large amounts of highly specific antibodies, generating high affinity murine antibodies revolutionized immunology as a field. The most important point with this approach is the adoption of innate functions of immune cells, maturated in lymphoid organs, through a humoral immune response and of cancers. The advent of hybridomas resulted from the convergence in the early 1970s of three technologies: (1) immortal myeloma–myeloma fusions; (2) mortal, antibody-producing B cells; and (3) assays to visualize antibody-producing B cells.
Myeloma-myeloma fusions secreting antibodies were generated at the Medical Research Council by César Milstein and his group. The fusion of an immortal murine myeloma cell line with human peripheral blood lymphocytes resulted in hybrid cell lines that continuously secreted human immunoglobulin was demonstrated by Schwaber and Cohen. Thus it is laid for the groundwork of specific hybridoma generation. OKT3 was one of a series of antibodies isolated by researchers after immunizing mice followed by screening for antigens on human T cells (OKT family), B cells (OKB family), and myeloid cells (OKM family). The murine anti-CD3 antibody, Orthoclone OKT3®, was produced by hybridoma culture.
There are several important points that should be stressed. First is the use of cancerous cells to confer B lymphocytes with immortality. Somatic fusion of B lymphocytes in spleen cells with cancerous myeloma cells generates hybridoma cells, which continuously produce antibodies in vitro. The other, is realization of the production of monoclonal antibodies specific to an epitope in an antigen.
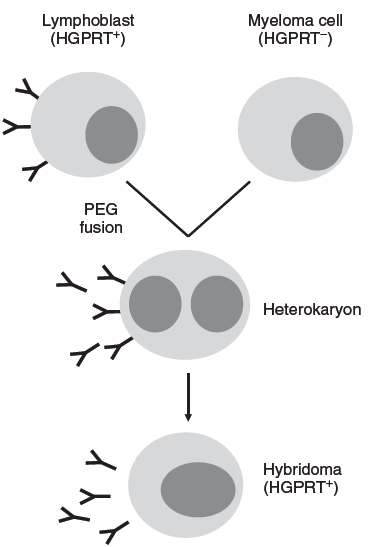
Conventional hybridoma production schedule might include primary immunization with antigen in Freund’s complete adjuvant, followed by boosts of antigen in incomplete Freund’s adjuvant at 2-3 weeks. Once enough titers against the antigen have been generated, the murine splenocytes are harvested and antibody-producing B cells (or, alternatively, B cells from lymph nodes and/or bone marrow, peripheral B cells) are harvested and fused to an immortal mouse myeloma cell lines such as NS0, SP2/0, or FO in the presence of either PEG. Plasma membrane fusion occurs, giving rise to a cell with two (or more) nuclei called heterokaryon. Hybridoma, or fused cell, have active HGPRT (the enzyme hypoxanthine-guanine phosphoribosyltransferase) from the B cells and grow in HAT medium, allowing for selective propagation of the newly formed hybridomas (Fig.1). Individual hybridoma clones are separated after selection so that each clone produces a single monoclonal antibody (mAb) with one specificity in a process known as subcloning. Antibodies from individual clones are then assayed for binding to the immunization antigen. Once desired antibodies are found, their V-regions are cloned from the hybridoma lines and made recombinant antibodies.
Fig.1 Principle of hybridoma production by cell fusion.(Stefan Dübel and Janice M. Reichert.2014)
There are two key aspects for successful production of novel mAbs based on hybridoma technology. The first aspect is immunization to facilitate differentiation of B lymphocytes into more matured forms. The second aspect is selective fusion of targeted antigen-sensitized B lymphocytes with myeloma cells. If these critical aspects are achieved, hybridoma technology can generate mAbs with high affinity and specificity, contributing not only to basic science research, but also to drug discovery and therapeutics on the basis of specific binding between antigens and antibodies.
The use of hybridoma technology to generate high affinity murine antibody revolutionized immunology as a field and has led to both the generation of tens of thousands of research tools as well as therapeutic candidate antibodies. However, the technology has obvious limiting factors for studies with substances which are not immunogenic in an animal host (e.g., macromolecules normally found in the host) or which cannot, for ethical reasons, be used to immunize humans.
Even if some species are difficult to be immunized, their monoclonal antibody can be produced by hybridoma. For example, guinea pig hybridoma production can be provided by Creative Biolabs which has extensive experience in various antibody production and engineering fields.