 As is known, antibody can be regarded as a specific protein that produced in response to counteracting an unique antigen, which is usually used to recognize and resist the invaders. It can combine chemically with the substances that the body recognizes as outsiders such as bacteria, viruses, and so on. Thus, people can reduce the levels of that special substance through introducing into the antibody. In addition, antibody is one of the most useful tools in biology and medicine. With the immune system, antibody can release a defensive protein, such as molecular markers for research of those destroyed diseased cells.
As is known, antibody can be regarded as a specific protein that produced in response to counteracting an unique antigen, which is usually used to recognize and resist the invaders. It can combine chemically with the substances that the body recognizes as outsiders such as bacteria, viruses, and so on. Thus, people can reduce the levels of that special substance through introducing into the antibody. In addition, antibody is one of the most useful tools in biology and medicine. With the immune system, antibody can release a defensive protein, such as molecular markers for research of those destroyed diseased cells.
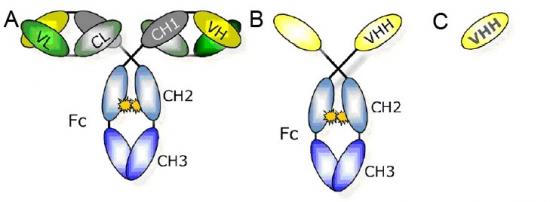
With the rapid development of antibody technology, nowadays single domain antibody can also accomplish the same task. And because its small body is accessible to the restricted area of macromolecules much easier, single domain antibody shows a more attractive prospect. However, there are still some difficulties to achieve this goal because scientists haven’t found an effective method to identify the single domain antibody. Fortunately, this problem has been solved by researchers from Rockefeller University; they have developed a new successful technology which can ensure that single domain antibody will significantly meet the needs of massive researching fields. The key is to find a relatively quick way to determine the gene sequence of high-affinity single domain antibody, because once those sequences are obtained it’s easy to conduct massive production of such single domain antibody utilizing bacteria.
Researchers first inoculate GFP and mCherry for the llamas, helping their immune system to fight against antibody of the two foreign proteins and then extracting RNA from llamas’ antibody-producing cells for the purpose of establishing a antibody sequence database. After follow-up several procedures, the most high-affinity antibody can be paired with those original RNA sequences with the help of computer program named “llama magic”. Based on this new sequence, researchers have produced 25 kinds of single domain antibodies that can precisely target at GFP and 6 kinds at mCherry, with a higher efficiency as well.
Furthermore, this new sequence has aroused a new inspiration: scientists can choose the most high-affinity single domain antibody and occasionally abandon those ungraded products, which can decline the required amount and then reduce the side effects to a better degree. To be brief, modern single domain antibody technology is able to identify high-affinity single domain antibody. Therefore, as a diagnostic and therapeutic tool, single domain antibody possesses a bright applicant prospect.