With Trump’s coming into power and the economic upheavals of Europe, the new drug approved by the FDA fell to a record low in 2016, following which the drug market of 2017 will be a difficult one. Even so, we have to believe that the policy of the drug market has always been on the right path. Looking back on the past 2016 and into 2017, let’s see what drugs may show themselves on the best-selling list of 2017.
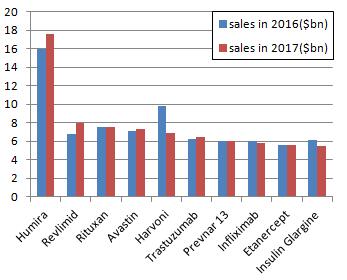
Adalimumab continues to lead other drugs. Over a period of time, its performance will not be surpassed by other drugs in the next few years. However, the growth rate of this biggest blockbuster has not been as shocking as ever. In 2017, the position of Gilead, once the upstart in drug industry, is quickly declining. Sales of hepatitis C drugs Harvoni will be lower than that of 2016. In the coming few years, the market will be reshuffled with the expiration of biological drugs patents.
1.Humira
Albergo’s Adalmu will continue to lead other drugs as the best-selling drug in 2017. Adalimumab is the world’s first approved anti-tumor necrosis factor TNFα drug. At the end of 2002, adalimumab, the FDA approved drug used in treating rheumatoid arthritis, went public in the United States. In 2003, EMA approved Adalimumab for the treatment of rheumatoid arthritis went public in the EU. In 2008, Abbott authorized Japanese Company Eisai to produce adalimumab which was approved for the treatment of rheumatoid arthritis and went public in Japan. In 2011, Adalimumab, the CFDA approved drug used in the treatment of rheumatoid arthritis, went public in the Chinese market. Adalimumab has been dominating the hegemonic position, leaving other drugs is the dust. It has maintained a strong growth momentum in recent years. It’s predicted that Adalimumab’s sales may exceed 18 billion by 2020. However, its growth rate will be gradually slowed down in the next few years, leading other drugs to compete with it. In July of 2016, Amgen’s Humira Biomedical Drug ABP501 was rated by the FDA’s Advisory Committee as “highly similar” to the original drug, making it possible to be approved. In December of 2016, CHMP supported the approval of Eli Lilly’s rheumatoid arthritis drug Baricitinib. In the trial of treating rheumatoid arthritis patients with Baricitinib, Baricitinib showed greater effects than Humira in terms of the two commonly used indicators.
2.Revlimid
Lenalidomide is an anti-tumor chemical developed by Celgene, which was approved by the FDA in 2005 under the trade name Revlimid. It is developed for the treatment of myelodysplastic syndrome (MDS). In 2013, the FDA approved lenalidomide’s supplemental New Drug Application for the treatment of mantle cell lymphoma (MCL) patients whose disease relapses or develops after been treated with two drugs (one of which is bortezomib).
3.Rituxan
Roche’s rituximab (Rituximab, trade name Rituxan) is used to treat autoimmune diseases and some cancers, especially non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, idiopathic thrombocytopenia Purpura, pemphigus vulgaris and other diseases. It was early developed by Nabil Hanna and its partners and was named IDEC-C2B8. They also applied patent (protection period lasts from 1998 to 2015) for it. Rituximab is a chimeric mouse / human monoclonal antibody. It was approved by FDA in 1997 for the treatment of relapse-resistant B-cell non-Hodgkin’s lymphoma, then approved by the European Commission in 1998 and listed in China in 1999.
4.Avastin
Roche’s Bevacizumab (trade name: avastin), approved by the FDA in 2004, is the first drug to be approved by the United States to inhibit tumor angiogenesis. It is also the first-line drug for the treatment of colon cancer. It gained approval by China in February of 2010. Combined with 5-fluorouracil-based chemotherapy, bevacizumab can be sued in the treatment of metastatic colorectal cancer.
5.Harvoni
Harvoni is a compound combination of Gilead’s highlight anti-hepatitis C drug Sovaldi (generic name: Sofosbuvir) and a fixed dose of protease NS5A inhibitor Ledipasvir. Enjoying tremendous popularity because of sovaldi’s excellent therapeutic effects, Gilead Sciences soon become the world’s top 10 pharmaceutical companies. However, due to the drug prices, pressure from competitive products and the hepatitis C market’s shrinking since 2015, Harvoni’s sales have been declining. Harvoni’s sale in 2016 was 9 billion US dollars, which declined 35.1% compared to that of 2015. In 2017, the sales will fall to $ 7 billion.
The other five drugs ranking from 6th to 10th will be introduced in the next report.
Source:
evaluatepharma
https://www.drugs.com/
https://www.fda.gov/