Immune cell therapy, the fourth major cancer treatment following surgery, radiotherapy, and chemotherapy, has been recognized as the most active and promising treatment in the comprehensive tumor treatment in the 21st century. It is the only hopeful means to completely kill the tumor cell nowadays. As the most effective cells in the human body to fight the tumor antigen-specific, T cells can pinpoint and target tumor cells. TCR service uses antigen-specific T cells to transfect the tumor specific TCR into the patient’s T cells to help identify and kill tumor cells T cells. This technology has a promising application prospect of solid tumors of which NY-ESO-1-specific TCR-T has achieved very significant results in the clinical practice of early solid tumor. For the patients with relapsed NY-ESO-1 positive adult T cell leukemia/lymphoma after HSCT, the allogeneic T cells transduced with a tumor-specific TCR by siTCR vector presented increased tumor-reactivity while GVHD potential is diminishing.
T cell receptor (TCR), a specific receptor on the surface of T cells, is responsible for identifying the antigen presenting by the major histocompatibility complex (MHC). Unlike the B cell receptor, TCR can not recognize free antigens. In general, T cell receptors have a lower affinity for the antigen, so that the same antigen may be recognized by different T cell receptors, and a receptor may also recognize many antigens.
There are three different T cell antigen receptors in vertebrates following as αβ TCR, γδTCR and pre-TCR. The pre-TCR is expressed only on the surface of immature αβ T cells while αβ TCR is expressed on the surface of mature αβ T cells and NK T cells, and γδTCR is expressed on the surface of γδT cells. ΑβTCR on the surface of the T cell recognizes the peptide primordia of the MHCI / II and the αβ TCR on the surface of the NK T cell recognizes the lipid antigen presented by CD1. However the ligand of the γδTCR is still not very clear, it can recognize MHC or MHC-like molecules (Such as mouse T22, T10, human CD1c) and also identify non-MHC molecules (such as viral glycoproteins, ATPase complexes). When recognizing the ligand, ΑβTCR and γδTCR will transmit the activation signal, stimulate T cell proliferation and secrete the corresponding cytokine. On the other hand, the transmission of the pre-TCR signal will not depend on the ligand. It spontaneously forms dimer on the cell surface to send activation signal and regulate T cells in the development of the thymus.
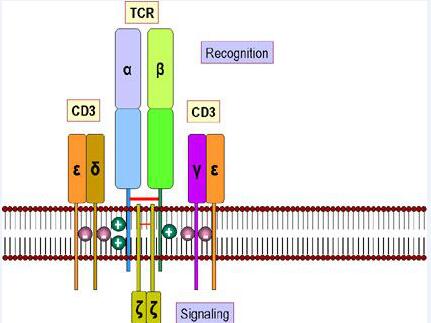
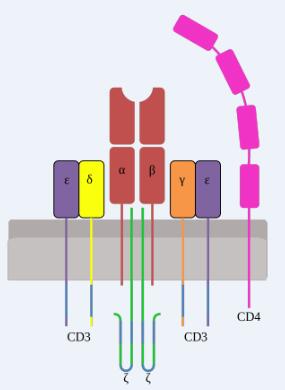
TCR is a typical marker for all T cell surfaces to identify the antigen, binding to CD3 with a noncovalent bond to form a TCR-CD3 complex. TCR, characterized by a very short cytoplasmic area, is a heterodimer composed of αand, two different peptide chain, which can be divided into the variable region (V region), constant area (C area), transmembrane regions and cytoplasmic areas and other parts.
TCR molecules belong to the immunoglobulin superfamily with its antigen-specific presence in the V region, where CDR3 has more variability than CDR1 and CDR2, directly determining the antigen binding specificity of TCR. When the MHC-antigen peptide complex is recognized by TCR, the CDRl, CDR2 recognizes and binds the sidewall of the MHC molecule antigen binding channel, and the CDR3 binds directly to the antigenic peptide.
MHC molecules on cells with antigen triggers the stimulation of TCR, initiating negative and positive cascades. These cascades eventually contribute to differentiation, cytokine production, cellular proliferation, and activation-induced cell death, regulating T-cell development, activation, homeostasis, and apoptosis.
TCR therapy not only can quickly kill the tumor like cytotoxic chemotherapy and targeted therapy but also can avoid the vaccine and T cell check point delay effect, achieving the transformation from the basic immunological mechanism to clinical immunotherapy application. Due to its expression of synthetic receptors and specifical recognization of target cells, TCR-T is becoming an aggressive cancer treatment and we are looking forward to more TCR T production.