All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Target Background
B-cell maturation antigen (BCMA) is a cell surface receptor encoded by TNFRSF17 gene and belongs to the TNF receptor superfamily. It is preferentially expressed in certain types of B cells including healthy plasma cells and has been shown to be of vital importance in B cell development and autoimmune response involving NF-kB and MAPK8/JNK activation. BCMA is an ideal target for immunotherapy as it is nearly uniformly expressed by the malignant plasma cells of most patients with Multiple Myeloma (MM) while has very low expression in other malignant or non-malignant tissues.
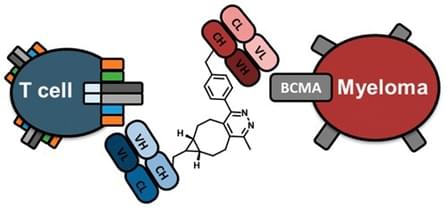
An Anti BCMA Bispecific Antibody for Multiple Myeloma
J. Am. Chem. Soc.2015,137 (16), pp 5288–5291
Anti-BCMA CAR-T Cell Therapy
Preclinical and early-stage clinical CAR-T based studies targeting BCMA have shown encouraging results. To date, six clinical trials (NCT02546167, NCT02215967, NCT02064387, NCT02325336, NCT02658929, NCT02786511) have been either initiated or under recruitment to further evaluate and promote clinical application of anti-BCMA CAR-T therapy. Particularly, data from a first-in-human study revealed promising effectiveness at the highest doses used, which assume one case of 17-week complete response before relapse and other good partial responses. The positive responses indicate great potential of anti-BCMA CAR-T therapy and also accelerate the research process to avoid severe toxicities suggestive of cytokine-release syndrome and prolonged cytopenia.
Animal Models for in vivo Study of anti-BCMA CAR-T Cell Therapy
Creative Biolabs aims to establish the top-ranking CAR-T in vivo study portfolio including pertinent models establishment to comprehensive efficacy and toxicity evaluation. Thereinto, techniques for the in vivo study of anti-BCMA CAR-T cell therapy for Multiple Myeloid are full-fledged.
Xenograft models for Multiple Myeloid
Typically, luciferase-labeled human myeloma cells OPM-2 are intravenously injected into female NSG mice. When the mean total flux of the tumor reaches between 107 and 108 photons/sec, mice are administered with BCMA-CAR-T cells or non-transduced control cells, intravenously. Tumor burden is monitored by in vivo imaging system.
CAR-T in vivo service platform at Creative Biolabs is designed to assist customers in touching the hotspot in bioresearch and to offer customers the most cutting-edge technologies in CAR-T in vivo research. Based on this principle, we are cooperating with multiple hospitals and institutions to establish more eminent types with good adaptability and clinical similarity such as patient-derived mouse models and humanized-xenograft models. Beyond that, any customized services are also welcome. Please feel free to contact our customer service for more information.
In vivo Assay Parameters and Techniques
At Creative Biolabs, we offer the most exquisite and comprehensive service platform for anti-BCMA CAR-T cell therapy research.
Efficacy Test
Tumor remission monitored by bioluminescence imaging and survival curve tracking
Viability and Bio-distribution Studies
Durability, GLP-compliant bio-distribution studies
Toxicity Evaluation
Pilot tolerability (MTD, the route of administration, Dose regimen / response / onset)
Clinical observation (body weight, feed consumption)
Cytokine storm surveillance (fever, hypertension, prolonged cytopenia)
Complete necropsy/organ weight
Tumorigenicity study
GLP-Compliant Preclinical Test
All our experiments are performed by well-trained and experienced technicians in a GLP-compliant and IACUC-regulated facility.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION