All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Target Background
Interleukin-3 receptor (CD123 antigen) is a heterodimer with a unique alpha chain paired and a common beta (CD131) subunit that belongs to the type I cytokine receptor family. Expressed in pluripotent progenitor cells, CD123 possesses the capacity to transmit IL-3 signal via cellular tyrosine phosphorylation and promote proliferation and differentiation of hematopoietic cells. Several hematologic neoplasms including acute myeloid leukemia (AML), hairy cell leukemia, plasmacytoid dendritic cell neoplasm, Hodgkin lymphoma and pediatric/adult ALL have been reported to express CD123 antigen. Besides, compared to lineage-associated surface antigens such as CD33 (myeloid) and CD19 (B lymphoid), CD123 is hierarchically expressed on hematopoietic progenitor cells as well as leukemic stem cells related to relapse and chemo-resistance in AML. Multiple modalities targeting CD123 such as IL-3 diphtheria toxin fusion protein, anti-CD123 mAbs and CD3Fv-IL-3 fusion construct have been developed, which, however, yielded minor clinical responses and thus suggested more powerful strategies like anti-CD123 CAR-T therapy to be tested.
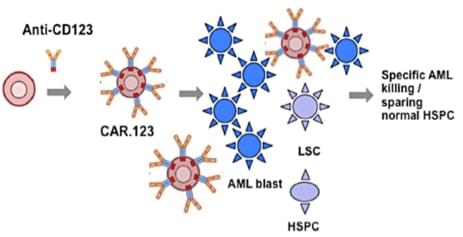
The strategy to eradicate AML cells via CAR immunotherapy
OncoImmunology. 2014 May 14; 3:e28835
Anti-CD123 CAR-T Cell Therapy
Several Phase I clinical trials (NCT02159495, NCT02623582) adopting anti-CD123 CAR-T therapy are now enrolling patients bearing AML of different stages. However preclinical studies have raised concerns for the use of anti-CD123 CAR-T without rescue strategy for myeloablation caused by the eradication of normal human myelopoiesis during treatment. Creative Biolabs possesses exquisite experiences on CAR design and subsequent in vivo assays, which fulfill requirements for preclinical researches on anti-CD123 CAR-T therapy as well as toxicity prevention-related studies.
Animal Models for in vivo Study of anti-CD123 CAR-T Cell Therapy
Xenograft models for systemic Acute Myeloid Leukemia (AML) are established via tail vein injection of luciferase-labeled human MOLM14 cells or KG1a cells into NSG mice. Disease progression can be traced by BLI. Creative Biolabs also provides patient-derived xenograft models, for which the leukemia engraftment will be defined as 1% human CD45+ cells in the peripheral blood by FACS. Anti-CD123 CAR-T cell therapy is given by tail vein injection of CAR-T cells or untransduced human T cells as control. Moribund or hind-limb paralysis is considered to be the endpoint of the in vivo study on AML models.
Xenograft models for systemic B cell acute lymphoblastic leukemia (B-ALL) are established by tail vein injection of luciferase-labeled human NALM 6 cells or primary B-ALL cells into NSG mice. Profound experience of CAR-T modification in Creative Biolabs enables the achievement of bispecific CAR-T cells targeting CD19/CD123 or CD33/CD123, which facilitates the improvement of CAR-T research in alliance with our all-around in vivo assays.
In addition to the referred xenograft models for AML and B-ALL, Creative Biolabs is also passionate about CAR-T in vivo study in any other CD123-related hematologic neoplasms such as Hodgkin lymphoma.
In vivo Assay Parameters and Techniques
At Creative Biolabs, we offer the most exquisite and comprehensive service platform for anti-CD123 CAR-T cell therapy research.
Efficacy Test
Tumor remission monitored by bioluminescence imaging (BLI)
FACS analysis
Survival curve tracking
Immuofluorescent microscopy
Viability and Bio-distribution Studies
Tumor infiltration, durability, GLP-compliant bio-distribution studies
Toxicity Evaluation
Pilot tolerability (MTD, the route of administration, dose regimen / response / onset)
Clinical observation (body weight, behavior, feed consumption)
Complete necropsy/organ weight
Tumorigenicity study
GLP-Compliant Preclinical Test
All our experiments are performed by well-trained and experienced technicians in a GLP-compliant and IACUC-regulated facility.
Creative Biolabs is willing to share our up-to-date service platforms as well as scientific expertise with our customers in order to realize a rational utilization of resource and a time-saving research flow. Please feel free to contact our customer service for detailed information.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION