All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Target Background
CD20 is a tetra-transmembrane protein expressed on almost all B cells from late pro-B phase (CD45R+, CD117+) to memory cells, excluding early pro-B cells, plasma blasts and plasma cells. More than 90% of B cell lymphomas, including acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), and hairy cell leukemias express CD20 antigen at a high copy number, and CD20+ cells are also found in some cases of Hodgkins disease, myeloma, and thymoma. CD20 is minimally internalized or shed. These features make CD20 an attractive target for the treatment of B cell neoplasms through immunotherapies. CD20 is the target of several clinically approved monoclonal antibodies (mAbs) such as rituximab, ibritumomab, and tositumomab. Numerous randomized trials have demonstrated improved clinical outcomes in patients treated with these mAbs, but it has been reported that some patients inevitably relapsed and developed resistance to repetitive antibody administration, despite that the majority of relapsed NHL B cells retain CD20 expression. CAR-T cell therapies targeting various tumor-associated antigens have shown unprecedented clinical efficacy, providing a potential alternative approach for treating B cell malignancies.
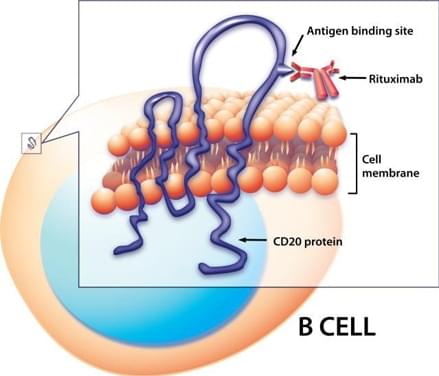
CD20 protein and CD20-targeted mAbs
June 2015, University of LausanneCHUV, Lausanne, Switzerland
Anti-CD20 CAR-T Cell Therapy
Data from several Phase I clinical trials have been reported. Patients with relapsed or refractory indolent B cell lymphoma or mantle cell lymphoma were treated with autologous T cells employing a anti-CD20 CAR. The anti-CD20 CAR-T cells were well tolerated and showed antitumor activity and minimal toxicity. In small numbers of patients, anti-CD20 CAR-T cell therapies even induced complete remissions. Another clinical trial of anti-CD20 CAR-T cell therapy with chemotherapy refractory advanced diffuse large B cell lymphomas obtained durable tumor regression in combination with lympho-depletion conditioning regimens in some patients. Further, early phase IIa trial in 11 patients with refractory or relapsed B-cell lymphoma confirmed the feasibility and efficacy of anti-CD20 CAR-T cell therapy. However, adverse effects including cytokine release symptoms, tumor lysis symptoms, aggressive intrapulmonary inflammation surrounding extranodal lesions and hemorrhage of the alimentary tract were observed.
Animal Models for in vivo Study of anti-CD20 CAR-T Cell Therapy
We have successfully developed various animal models for the in vivo assay of anti-CD 20 CAR-T cell therapy. Immunodeficiency mice are injected intravenously, subcutaneously, or intraperitoneally with CD20+ cells, such as lymphoma cell lines, CD20 transfected cell lines, human primary lymphoma cells, as well as customized cell lines to build up a murine B-cell malignancy model. Rat and non-human primate (NHP) models can be provided as well.
In vivo Assay Parameters and Techniques
At Creative Biolabs, we offer the most exquisite and comprehensive service platform for anti-CD20 CAR-T cell therapy research.
Efficacy Test
Tumor remission monitored by tumor cell analysis or bioluminescence imaging and survival curve tracking.
Viability and Bio-distribution Studies
Durability, GLP-compliant bio-distribution studies
Toxicity Evaluation
Pilot tolerability (MTD, The route of administration, Dose regimen/response/onset)
Clinical observation (body weight, feed consumption, ophthalmologic and clinical pathology)
Cytokine storm surveillance (fever, hypertension, prolonged cytopenia)
Complete necropsy, organ weight
Histopathology
Tumorigenicity study
GLP-Compliant Preclinical Test
All our experiments are performed by well-trained and experienced technicians in a GLP-compliant and IACUC-regulated facility.
Many factors, such as the composition of CAR, the transduction technology, the separation and expansion method of T cells may impact the results of anti-CD20 CAR-T cell therapy. Therefore, preclinical in vivo tests, determining the efficacy, toxicity, tolerability, and biodistribution characteristics are of great importance. As a leading biotech service provider, Creative Biolabs provides specialized, reliable, high-quality solutions for CAR-T development.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION