All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Target Background
CD33 or Siglec-3 (sialic acid binding immunoglobulin-like lectin 3) is a trans-membrane receptor, consisting of two extracellular immunoglobulin domains and two intracellular immunoreceptor tyrosine-based inhibitory motifs (ITIMs). The expression of CD33 is restricted in the myeloid lineage. It is highly expressed on myeloid progenitor cells but much lower on differentiated cells such as tissue macrophages and peripheral granulocytes. In addition, CD33 is highly expressed on, and considered as a marker of acute myeloid leukemia (AML), which is the most common form of leukemia in human. The prognosis of AML is very poor. Several studies have utilized CD33-targeted antibody-drug conjugate (ADC) such as vadastuximab talirine (developed by Seattle Genetics) to induce the lysis of myeloid leukemia cells in vivo.
Anti-CD33 CAR-T Cell Therapy
A preclinical test of anti-CD33 CAR-T cells showed significant effector functions in vitro, and induced reduction of leukemia burden and prolonged survival of AML xenograft murine models. However, the anti-CD33 CAR-T cell treatment resulted in serious hematopoietic toxicity in animal models, suggesting the permanent infusion of anti-CD33 CAR-T cells may yield unacceptable toxicity. Therefore, the novel 4th generation CARs that contain an "off switch" may be used to avoid long-term myelosuppression. Several clinical trials on relapsed and/or chemotherapy refractory CD33+ AML with autologous or allogeneic anti-CD33 CAR-T cells are being conducted. It is reported that adverse effect including fever, pancytopenia and elevated cytokine levels in serum are observed.
Animal Models for in vivo Study of anti-CD33 CAR-T Cell Therapy
To test the in vivo efficacy and safety of anti-CD33 CAR-T cells, SCID mice are injected with different kinds of CD33 positive cells such as MOLM 14 cell line or human primary AML cells via tail veins. Specially, to assess the hematopoietic toxicity of anti-CD33 CAR-T cells, mice employing humanized immune system that is myeloid biased have been used. We are also able to provide non-human primates (NHPs) and rats models.
In vivo Assay Parameters and Techniques
At Creative Biolabs, we offer the most exquisite and comprehensive service platform foranti-CD22 CAR-T cell therapy research.
Efficacy Test
Tumor remission monitored by tumor cell analysis or bioluminescence imaging and survival curve tracking.
Viability and Bio-distribution Studies
Durability, GLP-compliant bio-distribution studies
Toxicity Evaluation
Pilot tolerability (MTD, The route of administration, Dose regimen/response/onset)
Clinical observation (body weight, feed consumption, ophthalmologic and clinical pathology)
Cytokine storm surveillance (fever, hypertension, prolonged cytopenia)
Complete necropsy, organ weight
Histopathology
Tumorigenicity study
GLP-Compliant Preclinical Test
All our experiments are performed by well-trained and experienced technicians in a GLP-compliant and IACUC-regulated facility.
The therapeutic outcome of anti-CD33 CAR-T cells depends on many factors such as the CAR composition, the transduction method, the subtype and expansion method of T cells. It is essential to determine the efficacy, toxicity, tolerability and bio-distribution characteristics in animal models before clinical trials. With years of experience in developing cell therapy technologies, Creative Biolabs is able to provide a wide range of high-quality services for CAR-T preclinical in vivo assay. Various standardized and customized rodent models and assay systems have been developed. Also, assays in other species, including non-human primates (NHPs) are available.
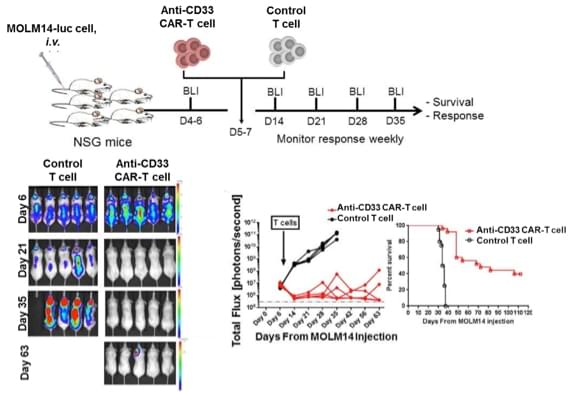
CAR-T33 treatment results in reduction in disease burden and prolonged survival in MOLM14-engrafted xenografts
Leukemia (2015) 29, 1637–1647
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION