All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Target Background
Epidermal growth factor receptor (EGFR), also known as ErbB-1, belongs to ErbB family of receptors. EGFR consists of 4 major parts, an extracellular domain, a hydrophobic transmembrane domain, an intracellular catalytic tyrosine kinase domain and several intracellular tyrosine residues. EGFR activation is closely associated with cellular growth and many publications suggest that EGFR is highly expressed in various solid tumors (e.g. breast, colon and non-small cell lung cancer) and thus it is considered as the first receptor directly related to human cancers. High expression level of EGFR is a potential diagnostic indicator and immunotherapeutic target of human carcinomas.
Anti-EGFR CAR-T Cell Therapy
For conventional antibody therapy, EGFR has been proved as a valuable target for monoclonal antibodies like cetuximab. However, efficacy of these anti-tumor agents is not very promising due to EGFR mutations. A Phase I/II clinical trial has been initiated to enroll patients with non-small cell lung cancer (NSCLC), cholangiocarcinoma and pancreatic cancer (NCT01869166), which are all EGFR positive. Patients receive dose escalating infusion of anti-EGFR CAR-T cells with or without chemotherapy. No therapy-related death is reported and the overall disease control rate is around 79% (19 out of 24).
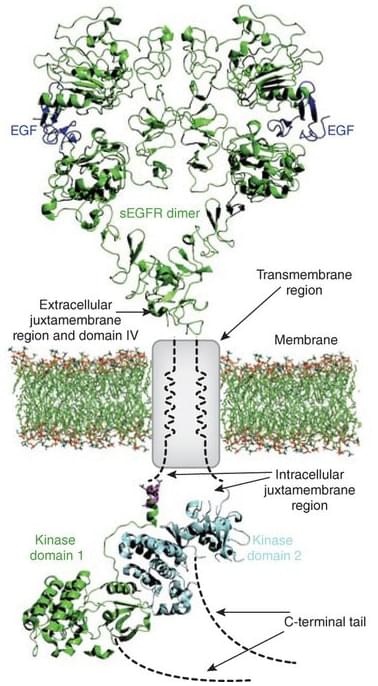
Finding the missing links in EGFR.
Nature Structural & Molecular Biology, 2012. 19(1), 1.
Animal Models for in vivo Study of anti-EGFR CAR-T Cell Therapy
Creative Biolabs has established many in vivo tumor models for testing anti-EGFR CAR-T cells. All animals are maintained in a clean and feed enriched environment before experiments. Animal experiments are monitored under an approved Institutional Animal Care and Use Committee (IACUC) protocol.
Xenograft model of NSCLC
Human NSCLC cell A549 are prepared in serum- and antibiotic-free medium and subcutaneously injected in scapular region of NOD/SCID mice. CAR-T cells are injected intravenously to mice after tumor reach 500mm3.
Xenograft model of mesothelioma
EGFR positive M108 cells derived from primary pleural effusion of mesothelioma patient are subcutaneously injected into NOG mice M108. Tumors are allowed to grow 6 to 7 weeks and engineered CAR-T cells are intravenously injected.
In vivo Assay Parameters and Techniques
Creative Biolabs offers comprehensive services to evaluate anti-EGFR CAR-T cell therapy. Our technical group is very experienced and efficient in data production. Experimental techniques can be customized at clients' requests or purpose-orientated.
Efficacy Tests
Tumor remission monitored by tumor volume recording or bioluminescence imaging and survival curve tracking.
Viability and Bio-distribution Studies
Durability and bio-distribution are evaluated by bioluminescence imaging, immunochemistry staining and real-time PCR
Toxicity Evaluation
Pilot tolerated evaluation: route of administration, dosage, MTD
Clinical observation: body weight, food consumption, behavior and pathological signs
Cytokine storm surveillance (fever, hypertension, prolonged cytopenia)
Postmortem analysis
Tumorigenicity study
GLP-Compliant Preclinical Test
All our experiments are performed by well-trained and experienced technicians in a GLP-compliant and IACUC-regulated facility.
Creative Biolabs is highly experienced and equipped with state-of-the-art facilities. We fully understand that researches of anti-EGFR CAR-T cell therapy is a time-consuming and costly process. We would like to provide a wide range of services to assist you and your team to accelerate this process.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION