All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Target Background
MUC16, well known as CA125 (carbohydrate antigen 125 or cancer antigen 125), is a member of the mucin family glycoprotein ranked as the largest membrane-associated mucin with about 22,000 amino acids, which is initially detected by the murine monoclonal antibody designated OC125 (the 125th Ab produced against the ovarian cancer cell line) and named " cancer antigen 125". It consists of a cytoplasmic tail for the interaction with cytoskeleton, a transmembrane domain and several extracellular domains including a highly O-glycosylated N-terminal domain, a tandem repeat (high in serine, threonine and proline) and multiple SEA (sea urchin sperm protein, enterokinase and agrin) modules, which contain a proteolytic cleavage site for release of extracellular region from the cell surface. Normally, the physiologic distribution of MUC16 is restricted on the ocular surface including the cornea and conjunctiva, the respiratory tract and the female reproductive tract epithelia, acting as a lubricating barrier against foreign particles and infectious agents via a hydrophilic environment created by the dense glycosylation. The expression of MUC16 is significantly changed in dry eye, cystic fibrosis and several types of cancers. MUC16 is the most frequently used biomarker for ovarian cancer detection recommended by American Congress of Obstetricians and Gynecologists and UK's National Institute for Health and Clinical Excellence (NICE). Meanwhile, MUC16 is also used as a marker for endometrial cancer, fallopian tube cancer, non-small cell lung cancer, breast cancer, pancreatic cancer and gastrointestinal cancer. Thus, MUC16 plays a critical role in advancing tumorigenesis and tumor proliferation, which manifests it as a target for immunotherapy of a great potential. Creative Biolabs helps researchers initiate the anti-MUC16 CAR-T cell therapy.
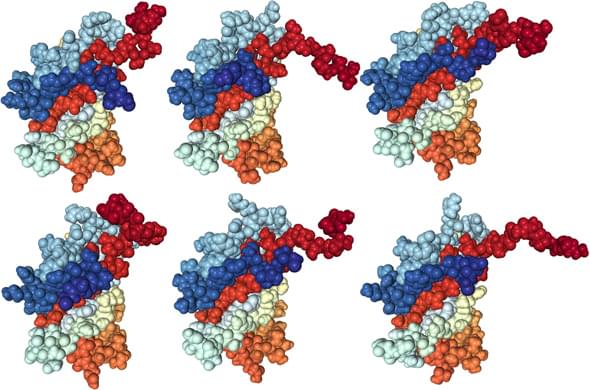
Different postures of the SEA domain from murine hypothetical protein homologous to human mucin 16
Journal of Biological Chemistry 279.13 (2004): 13174-13182
Anti-MUC16 CAR-T Cell Therapy
A phase 1 clinical trial (NCT02498912) with cyclophosphamide followed by intravenous and intraperitoneal infusion of autologous T cells (4H11-28z/fIL-12/EFGRt+ genetically modified to secrete IL-12 and to target the MUC16, which is present on about 70% of ovarian cancers) in patients with recurrent MUC16+ epithelial ovarian, fallopian tube or primary peritoneal cancer after standard chemotherapy has completed. Other pre-clinical or clinical studies targeting MUC16 are not reported up to now. Thus, the miraculous anti-MUC16 CAR-T cell therapy offers researchers an incredible chance to find values in this unexpected place. With the help of Creative Biolabs, the brighter and prosperous future of anti-MUC16 CAR-T cell therapy is nearly at researcher's hand.
Animal Models for in vivo Study of anti-MUC16 CAR-T Cell Therapy
Creative Biolabs offers researchers almost all the animal models generated from the major techniques including the conventional carcinogen-induced models, the mosaic transposon- or virus-induced models, the transgenic models and the subcutaneous or patient-derived xenograft models for a variety of ovarian cancer and other malignancies such as endometrial cancer, fallopian tube cancer, non-small cell lung cancer, breast cancer, pancreatic cancer and gastrointestinal cancer. Furthermore, Creative Biolabs is always passionate about introducing our well-established animal models to in vivo tests of adoptive cell therapy and providing assistance in creating the most clinically relevant animal models with customized requirements.
In vivo Assay Parameters and Techniques
At Creative Biolabs, we offer the most exquisite and comprehensive service platform for preclinical MUC16 CAT-T cell therapy research.
Efficacy Test
Tumor remission monitored by tumor volume recording or bioluminescence imaging and survival curve tracking
Viability and Bio-distribution Studies
Durability, GLP-compliant bio-distribution studies
Toxicity Evaluation
Pilot tolerability (MTD, The route of administration, Dose regimen/response/onset)
Clinical observation (body weight, feed consumption, ophthalmologic and clinical pathology)
Cytokine storm surveillance (fever, hypertension, prolonged cytopenia)
Complete necropsy, organ weight
Histopathology
Tumorigenicity study
To sublimate the fundamental capabilities of CAR-T cell therapy to a new level, Creative Biolabs provides the most scientific in vivo pre-clinical assay design with the most clinically relevant animal models via thinking the first principles instead of formulas to write the prosperous future of CAR-T cell therapy from brief scratch.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION