All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Target Background
>Natural-killer group 2, member D (NKG2D), a transmembrane protein belonging to the CD94/NKG2 family of C-type lectin-like receptors, is a prime recognition receptor for the detection and elimination of transformed and infected cells. It functions by binding its ligands that are induced during cellular stress such as infection or genomic stress occurred in cancer. In human, two classes of NKG2D ligands are involved, the MHC class I related antigens A and B (MICA and MICB) and the UL-16 binding proteins (ULBP1-6). The fact that NKG2D ligands are induced self-proteins that are completely absent or expressed in low levels in normal cells compared to the high expression levels in the infected, transformed, senescent and stressed cells renders it a potential target for CAR-T therapy. Cancer cells with high level NKG2D ligands are susceptible to T/NK cell-mediated lysis.
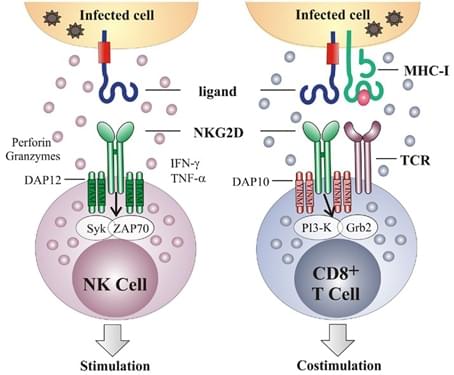
NKG2D acts as an activating and a co-stimulatory receptor
Front Immunol. 2011 Dec 28; 2:85.
Anti-NKG2D-ligand CAR-T Cell Therapy
NKG2D ligands are expressed in many primary tumor cells and the NKG2D based CARs have the potential to recognize approximately 90% of human tumor types, which prompts NKG2D ligands to be an attractive target for adoptive T cell therapy. At the same time, these ligands are also induced under a variety of physiological circumstances, which raises concerns about "on-target off-tumor" toxicity. For this reason, many more preclinical studies need to be conducted for the improvement of NKG2D ligand CAR-T therapeutic efficacy and also for the precaution of toxicity in clinical trials. Creative Biolabs has gained extensive in vivo assay experience and will offer our customers the best service to assure your research improvement at each and every step.
Animal Models for in vivo Study of anti-NKG2D-ligand CAR-T Cell Therapy
Xenograft models for ovarian cancer
At Creative Biolabs, we provide service for the establishment of the syngeneic mouse models derived from the murine ovarian cancer cell line ID8 (luciferase-labeled) for anti-NKG2D ligand CAR in vivo study in both C57BL/6 and BALB/c mice.
Xenograft models for other malignances
Targeting NKG2D ligands by NKG2D CAR-T cells has advanced to Phase I clinical trials in patients with AML, MDS-RAEB and Multiple Myeloma. Optimization of CAR structure often results in correspondingly better efficacy. Thus, Creative Biolabs aims to assist our customers in the in vivo exploration of any NKG2D ligands-related cancers taking advantage of our world-wide animal resources, extensive cooperation backgrounds and outstanding research team.
In vivo Assay Parameters and Techniques
At Creative Biolabs, we offer the most exquisite and comprehensive service platform for anti-MUC1 CAR-T cell therapy research.
Efficacy Test
Tumor remission monitored by tumor volume recording or bioluminescence imaging and survival curve tracking
Viability and Bio-distribution Studies
Durability, GLP-compliant bio-distribution studies
Toxicity Evaluation
Pilot tolerability (MTD, the route of administration, dose regimen/response/onset), Clinical observation (body condition, body weight, feed consumption)
Cytokine storm surveillance (multiplex cytokine array)
Complete necropsy, organ weight
Lung histopathology (perivascular edema, diffuse thickening of the alveolar septa, neutrophils and mononuclear cells infiltration)
Tumorigenicity study
GLP-Compliant Preclinical Test
All our experiments are performed by well-trained and experienced technicians in a GLP-compliant and IACUC-regulated facility.
Creative Biolabs intends to break through the limits in line with conventional animal models and to explore more eminent types presenting good adaptability and clinical similarity such as patient cell derived mouse models and humanized-xenograft models (humanized TRAMP/MIC mice, a powerful tool for validating various targeting strategies for MIC+ malignancies) in order to meet the rigorous requirement of CAR-T study. Beyond that, any customized services are also welcome. Please feel free to contact our customer service for more information.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION