All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Target Background
Wilms'tumor 1 (WT1) encodes a transcription factor consisted with four zinc-finger motifs at the C-terminus and a proline/glutamine-rich DNA-binding domain at the N-terminus, which physiologically plays an essential role in the cell growth and differentiation, especially in the normal development of the urogenital system. In the matter of biological distribution and related pathology, WT1 is expressed in the normal mammary duct and lobule and mutated in a subset of patients with Wilms'tumor for the namesake and causes an embryonic malignancy of the kidney with the incidence of 1 in 10,000 infants. Although the WT1 was originally identified as a tumor suppressor, the subsequent reports demonstrated the significant high expression of WT1 in various hematological malignancies such as acute myelocytic and lymphocytic leukemia, chronic myelocytic leukemia and solid tumors including the gastrointestinal and pancreaticobiliary system, urinary tract, male and female genital organs, breast, lung, brain, skin, soft tissues and bone. Furthermore, the WT1 has been demonstrated not only as a diagnostic but also a robust prognostic marker in acute myeloid leukemia, colorectal cancer, breast cancer and high-grade serous ovarian carcinoma. In addition, the WT1 is ranked by the National Cancer Institute (NCI) as the No. 1 target for cancer immunotherapy. Therefore, the WT1 manifests great potentials as a target for CAR-T cell therapy.
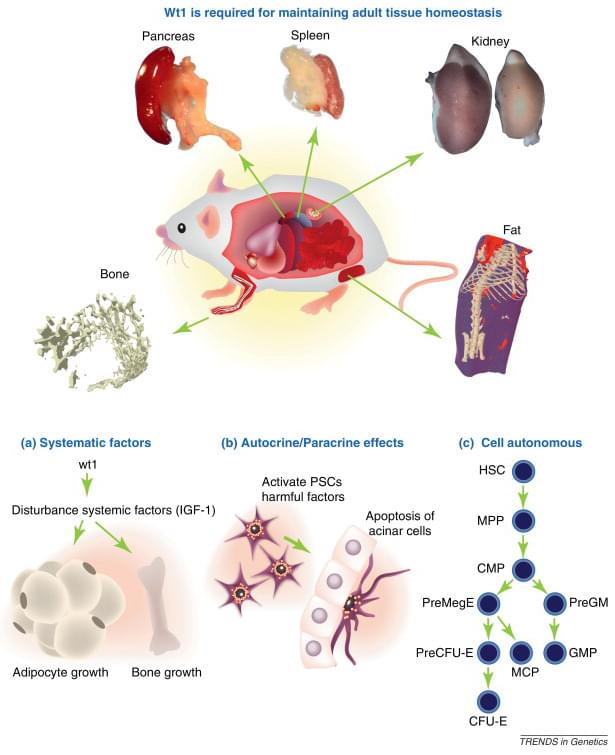
Physiological function of WT1 for maintaining adult tissue homeostasis
Trends in Genetics 28.10 (2012): 515-524
Anti-WT1 CAR-T Cell Therapy
As for WT1 immunotherapy, multiple phase1/2 clinical trials have been completed for determining the safety, effectiveness and side effects of WT1 vaccine (NCT00923910), the WT1 antigen-specific cancer immunotherapeutic (WT1 ASCI) as post-induction therapy (NCT01051063) or post-consolidation (NCT00725283) therapy in multiple WT1-positive hematological malignancies, including AML, ALL, CML, etc. However, there is still no effort made for anti-WT1 CAR-T cell therapy via WT1 target currently. Creative Biolabs helps acute researchers break all the obstacles of pre-clinical study, and occupy the virgin land of anti-WT1 CAR-T cell therapy.
Animal Models for in vivo Study of anti-WT1 CAR-T Cell Therapy
Creative Biolabs offers almost all animal models for the preclinical in vivo studies for all kinds of hematological malignancies such as acute myelocytic and lymphocytic leukemia and chronic myelocytic leukemia, and solid tumors including the gastrointestinal and pancreaticobiliary system, urinary tract, male and female genital organs, breast, lung, brain, skin, soft tissues and bone. Take leukemia model as an example:
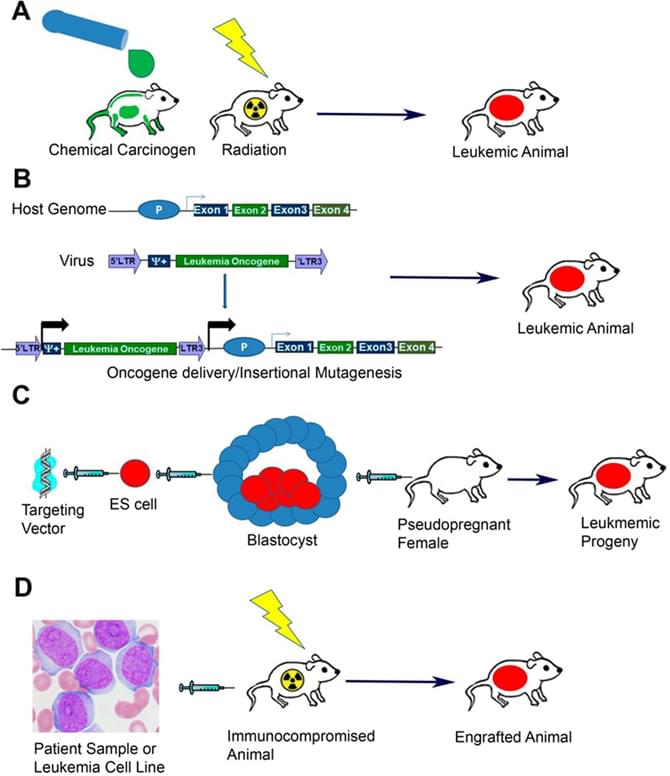
The major techniques for generating animal models of leukemia
Cancer and Metastasis Reviews 32.1-2 (2013): 63-76
Furthermore, Creative Biolabs provides assistance in creating clinically relevant animal models with customized requirements.
In vivo Assay Parameters and Techniques
At Creative Biolabs, we offer the most exquisite and comprehensive service platform for preclinical WT1 CAT-T cell therapy research.
Efficacy Test
Tumor remission monitored by tumor volume recording or bioluminescence imaging and survival curve tracking
Viability and Bio-distribution Studies
Durability, GLP-compliant bio-distribution studies
Toxicity Evaluation
Pilot tolerability (MTD, The route of administration, Dose regimen/response/onset)
Clinical observation (body weight, feed consumption, ophthalmologic and clinical pathology)
Cytokine storm surveillance (fever, hypertension, prolonged cytopenia)
Complete necropsy, organ weight
Histopathology
Tumorigenicity study
To unceasingly offer researchers convenience and assistance with the rapid scientific and technological progress, Creative Biolabs is dedicated to establishing the most reliable and relevant animal models in order to meet the rigorous requirements of preclinical studies for IND application. Beyond that, we are pleased to design studies together with our customers and provide customized service. Creative Biolabs fuels your research voyage with next century's pace. Please feel free to contact our customer service for more information.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION