Through the advanced CreDA™ platform, Creative Biolabs provides an efficient immunogenicity assessment for biotherapeutic candidates, both in silico prediction and in vitro assays are included in this platform to guide customers to select drug candidate with lower immunogenicity risk.
Immunogenicity is the inherent ability of protein drugs to induce humoral or cell-mediated immune responses. Many drug candidates are frequently found to be immunotoxic and induce ADA responses in clinical trials, which can lead to allergic reactions, reduction or neutralization of drug activity, cross-reactive immune responses, or even more serious adverse events. Based on these facts, the immunogenicity of protein drug is often considered to be one of the primary causes for attrition during early clinical development. To avoid such failure, it's of great importance to perform immunogenicity assessment during the developability assessment stage, which can help pharmaceutical company select candidate with the lowest risk of generating an unwanted immune response in the clinical trial, thus sharply lowering the development cost.
Immunogenicity assessment module in our CreDA™ platform
Given the rich experience in bioinformatics and immunology, our researchers have combined both in silico prediction and in vitro assays to develop an advanced assay system for efficient immunogenicity assessment of drug candidates. This system is now integrated as an important module of the CreDA™ platform. These assays mainly involve in silico immunogenicity prediction, in vitro antigen presentation assay, and DC-T assay, which could be combined to identify drug candidates with potential immunogenicity risk. Furthermore, based on the identified epitope sequence, de-immunization service is also supplied and unwanted immunogenicity can be migrated by substituting key residues in the epitope peptide that do not affect drug potency.
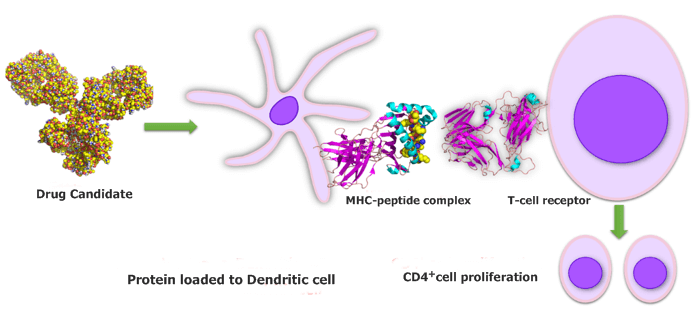 Fig.1 DC-T cell assay of our CreDA™ platform to assess the drug immunogenicity.
Fig.1 DC-T cell assay of our CreDA™ platform to assess the drug immunogenicity.
In silico immunogenicity assessment
With rich experience in bioinformatics, our researchers have developed a structure-based immunogenicity prediction system, which could model the 3D structure of the peptide-MHC (pMHC) complex with high accuracy, allowing the prediction of potential immunodominant epitopes at allele-specific level without the need of large experimental data set for training. Peptide structure in the MHC binding groove is modeled using an advanced docking algorithm and energy function. The binding affinity of peptides to MHC allele is evaluated and ranked by free energy calculation. Peptides with high binding affinity are predicted as binder of the MHC molecule and thus may be potential T-cell epitopes and increase the in vivo immunogenicity risk of drug candidates.
DC-T cell assay for whole protein screening
Dendritic cell (DC)-T cell assay is developed to measure if a whole protein drug could induce helper CD4+ T cell proliferation, which may lead to ADA response or other unwanted immunogenicity. It allows for an overall comparison of the T cell-driven antigenicity of any number of candidates at the developability assessment stage. Besides, it can also be used for assessing the impact on antigenicity of factors other than protein sequence, such as protein modifications, degradation products, excipients, or drug formulation. The DC-T cell assay uses blood samples from healthy high-resolution MHC Class II tissue-typed donors. And a donor cohort can be selected to provide representative coverage of a particular population of interest and to mimic the allele frequencies found in the global population.
Antigen presentation assay
Antigen presentation assay aims to the direct identification of the peptide fragment of a drug candidate that can be presented to T cells by the MHC molecules on dendritic cells, which is the most direct way for measuring antigen processing and presentation. It could also be used to compare different drug formulations and the effect of donor MHC types and is compatible with fully-formulated biologics. Binding peptides are identified through a workflow mainly consisting of MHC-peptide complex extraction, peptide elution and peptide sequencing by mass spectrometry. Antigen presentation assay can be used to identify the peptide epitopes associated with MHC-DR, DP, and DQ alleles.
Immunogenicity assessment can identify the drug candidates with potential in vivo immunogenicity risk, and thus de-risk the molecule selection in the early development stage. If you are interested in this service, please contact us for more information and a detailed quote.
For Research Use Only.
 Fig.1 DC-T cell assay of our CreDA™ platform to assess the drug immunogenicity.
Fig.1 DC-T cell assay of our CreDA™ platform to assess the drug immunogenicity.