- Home
- ADC Development
- Antibody Design & Conjugation for ADC Development
- EnCys-mAb based Conjugation Strategy
EnCys-mAb based Conjugation Strategy
Creative Biolabs assembles a professionally trained science team to generate thiol-engineered antibody (EnCys-mAb) for site-specific conjugation in antibody-drug conjugates (ADCs) production. Our extensive experience in antibody engineering and ADC development allows us to provide top quality EnCys-mAb to meet your homogeneous ADC development goals.
EnCys-mAb
EnCys-mAb, also known as thiol-engineered antibody, refers to antibodies with cysteine substitutions at specific sites where those engineered cysteines are available for conjugation but do not affect immunoglobulin folding, antibody assembly, antigen binding, and Fc domain effector functions. Engineered cysteines can be introduced into the amino acid sequence by site-directed mutagenesis. Typically, two engineered cysteines are introduced per antibody for payload drug conjugation and the resulted ADCs usually exert a narrow drug to antibody ratio (DAR) at 2. For the identification of potential cysteine insertion sites, phage display technique can serve as a good tool and previous studied have demonstrated that the ideal sites for residue substitution include eight residues on the light chain (LC-V205C, LC-S168C, LC-A153C, LC-S127C, LC-S121C, LC-S114C, LC-V110C, LC-V15C) and five residues on the heavy chain (HC-T116C, HC-S115C, HC-A114C, HC-S113C, HC-S112C), respectively.
EDC
The ADCs developed based on EnCys-mAb technique are known as EnCys-mAb Drug Conjugates (EDCs). Generally, EDCs are produced following a process involving sequential reduction, re-oxidation, and conjugation. Firstly, a global reduction step uncaps blocked free cysteine residues and breaks inter-chain disulfides of a EnCys-mAb. A subsequent oxidation step follows and in the presence of CuSO4 or dehydroascorbic acid, the inter-chain disulfide bonds are regenerated, while the thiol groups on the engineered Cys residues remain free. Finally, payload drugs are conjugated to the engineered Cys residue reactive thiol groups via maleimide-containing linkers to generate EnCys-mAb Drug Conjugates. It has been reported that using optimized reduction conditions, ADCs conjugated through EnCys-mAb can achieve over 98% conversion rate, most of which bearing a DAR of 2.0. The high efficiency and controlled specificity makes EnCys-mAb more desirable for both laboratory and industrial scale ADC production.
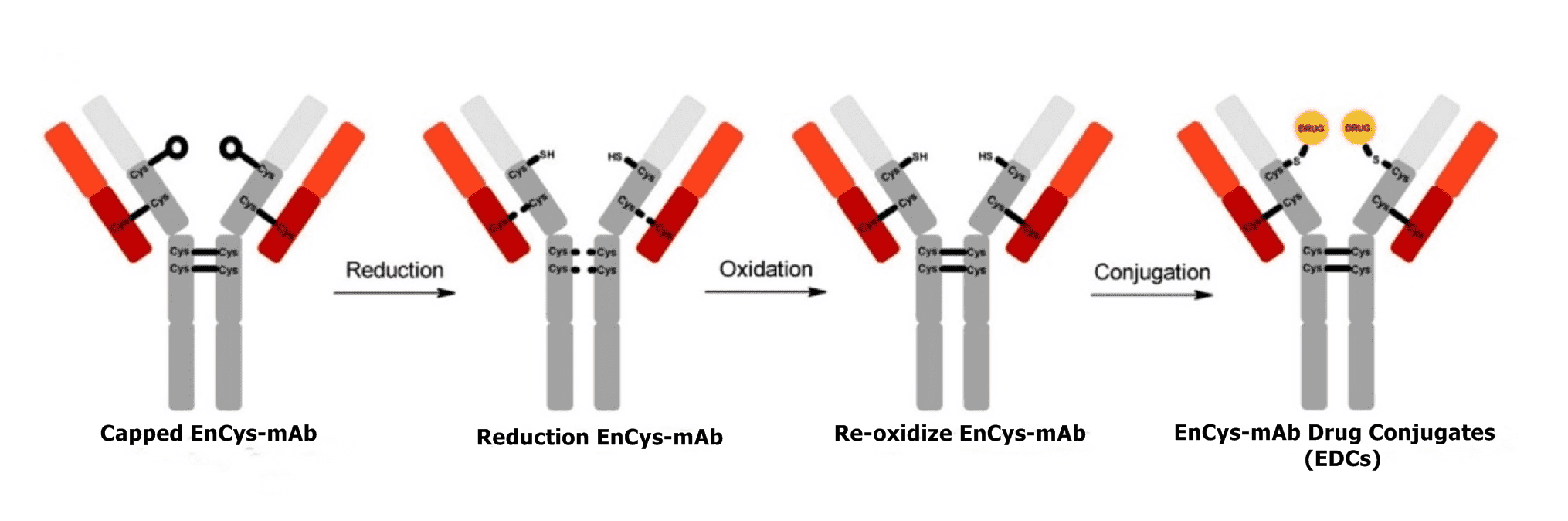 Fig.1 Generating EnCys-mAb Drug Conjugate.1,2
Fig.1 Generating EnCys-mAb Drug Conjugate.1,2
Advantages of EnCys-mAb-based conjugation:
- This strategy generates high purity, conjugation-site specific ADCs with a DAR of 2.0
- The EnCys-mAb-based conjugation produces more homogeneous ADCs with a well-defined and improved pharmacokinetic profile
- This strategy is suitable for the conjugation to both Fab and full length IgG
- EnCys-mAb has no significant influence on antibody structure, antigen binding affinity, antibody specificity, and conjugate stability
- This strategy is easily amplifiable for large scale manufacture of homogeneous ADC with consistent conjugate sites and DARs
Creative Biolabs provides various antibody modification and conjugation strategies in a timely and cost efficient manner. We are dedicated to provide our clients with high-quality services and contribute to your ADC development projects. Please contact us for more information and a detailed quote.
References:
- Sochaj, Alicja M., Karolina W. Świderska, and Jacek Otlewski. "Current methods for the synthesis of homogeneous antibody–drug conjugates." Biotechnology advances 33.6 (2015): 775-784.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
