- Home
- Resources
- Knowledge Center
- Literatures
- A Two-Step Immunocapture Assay for ADCs Characterization
A Two-Step Immunocapture Assay for ADCs Characterization
Thiol-Maleimide-Based ADCs
Plasma stability assessment is a key step in developing and optimizing ADCs, especially those with cleavable linkers. The generation of free payloads from a cleavable linker in plasma is easily monitored by LC/MS. Further steps can be taken to capture the remaining ADCs in plasma and cleave the residual payload to determine the amount of antibody-conjugated payloads. However, thiol-maleimide-based ADCs can undergo deconjugation of the linker payload through a thiolexchange process with cysteines on plasma albumin, which results in the loss of payload from ADCs. To optimize the stability of thiol-maleimide-based ADCs, a screening assay is needed to assess overall ADC plasma stability by simultaneously deconvoluting the stability of both the linker and payload.
In this study, a novel two-step immunocapture LC/MS/MS approach is described to allow the quantification of conjugated payloads, total antibodies, and migrated payloads forming adducts with albumin in the plasma samples for stability assessment. The ADC stability assessment was illustrated using TAK-001, a proprietary payload, with a maleimide linker-based ADC.
Our maleimide linker products
| CAT | Product name | CAS NO | Molecular Weight | Purity |
|---|---|---|---|---|
| ADC-L-663 | Maleimide-C6-amine TFA salt | 731862-92-3 | 196.3 | 95% |
| ADC-L-666 | Maleimide-heptanoic NHS ester | 55750-63-5 | 308.3 | 95% |
| ADC-L-671 | Maleimide-PEG4-Glu(OH)-NH-m-PEG24 | - | 1615.9 | 95% |
| ADC-L-656 | Maleimide-amido-PEG-methyl (PEG1-PEGn) | 1263044-81-0 | 358.4 | 95% |
| ADC-L-600 | Biotin-PEG-maleimide (PEG1-PEGn) | 305372-39-8 | 525.6 | 95% |
| ADC-L-683 | Tri(Maleimide-PEG2-amide)-amine | - | 1077.2 | 95% |
Two-Step Immunocapture
In two-step immunocapture methods, magnetic beads were used to extract the target antigen, ADC, and antibody-associated species. The remaining liquid was then further extracted using anti-albumin beads to collect the albumin-associated adducts for analysis. Specifically, 10 μL of each stability sample was added to a 96-well plate containing 0.1 mg of target antigen coupled M-280 beads suspended in PBST solution. The samples and beads were mixed and incubated for 40 minutes at room temperature. After the first extraction cycle, the magnetic beads were collected in PBST solution. A second extraction cycle was performed using the remaining supernatant and another 0.1 mg of target antigen coupled M-280 beads. The beads from both cycles were separately washed with PBST solution, PBS, and 10% acetonitrile before being released into a papain activation solution. After depleting the ADCs, approximately 200 μL of the remaining liquid was mixed with prewashed PureProteome Albumin Magnetic Beads and incubated for 60 minutes with rotation. The supernatants were then transferred to new tubes, and protein precipitation was performed by adding acetonitrile with formic acid. The anti-albumin magnetic beads in the microcentrifuge tubes were washed, and all of the resulting protein pellets and magnetic beads were digested with papain.
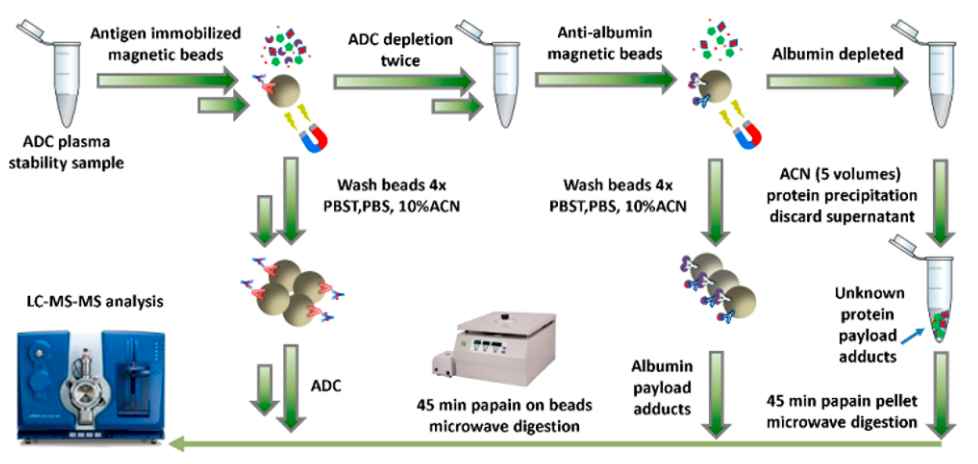 Fig. 1. Bioanalytical workflow for quantifying plasma albumin payload adducts by ADC depletion followed by anti-albumin magnetic bead enrichment.1
Fig. 1. Bioanalytical workflow for quantifying plasma albumin payload adducts by ADC depletion followed by anti-albumin magnetic bead enrichment.1
Quantitation of Free Payload, Total Antibody, Conjugated Payload, and Deconjugated Payload.
Discrete single-step immunocapture LC/ MS/MS assays are used to measure total antibody and conjugated payload concentrations on days 0, 1, 2, 3, and 6.
- As shown in Figure 2a, the free payload, TPP, was stable in human plasma for up to 6 days and moderately stable in mouse plasma for at least 3 days.
- As shown in Figure 2b, the total antibody exhibited remarkable stability when incubated with human and mouse plasma at 37 °C for up to 6 days. However, only approximately 80% of the total payload, which includes both conjugated and deconjugated payloads in mouse/human plasma, could be retrieved from the immunocaptured ADC (as shown in Figures 2c and 2d).
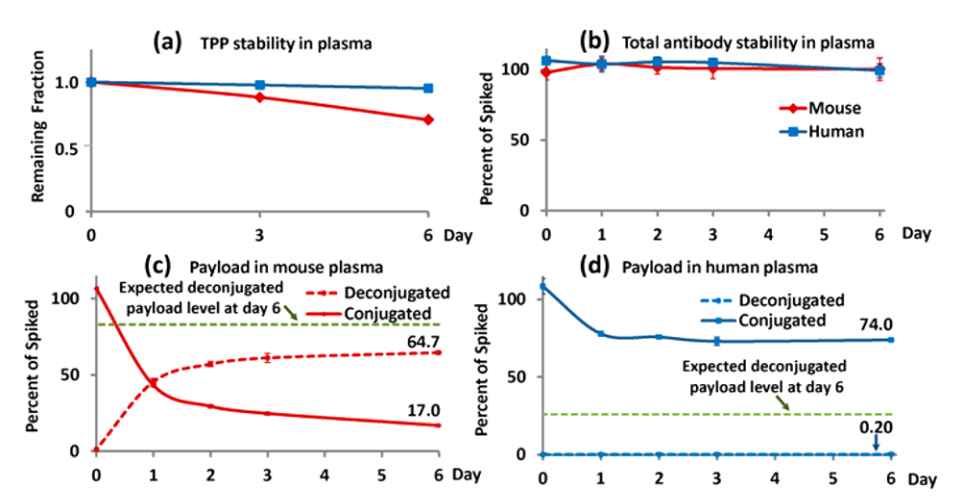 Fig. 2. In vitro plasma stability profiles of free payload, total antibody, conjugated payload, and deconjugated payload.1
Fig. 2. In vitro plasma stability profiles of free payload, total antibody, conjugated payload, and deconjugated payload.1
Quantitation of Payload Adducts with Plasma Albumin and Other Proteins.
A two-step immunocapture (Figure 1) is used to measure the potential loss of conjugated payload in each fraction.
- In the day 6 mouse plasma sample, the levels of TPP-associated albumin and pellet-associated linker-payload adducts are 13.6 and 0.4 ng/mL, respectively.
- In human plasma, the TPP levels of albumin- and pellet-associated linker-payload adducts are found to be 16.9 and 1.4 ng/mL, respectively.
Taking into account the thiol exchange, the above results indicate that most of the payload could be recovered from the day 6 plasma stability sample in both species by two-step immunocapture.
Creative Biolabs has extensive experience in developing ADCs and serves as your one-stop solution provider for thiol-maleimide-based ADC in vitro analysis and characterization.
Reference
- Dong, Linlin, et al. "A two-step immunocapture LC/MS/MS assay for plasma stability and payload migration assessment of cysteine-maleimide-based antibody drug conjugates." Analytical chemistry 90.10 (2018): 5989-5994.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
