- Home
- ADC Development
- ADC In Vitro Analysis
- ADC Biochemical Analysis
- ADC Stability Analysis
- ADC Chemical Stability Analysis
ADC Chemical Stability Analysis Service
The chemical stability of an antibody-drug conjugate (ADC) is contributed by its individual components (the monoclonal antibody, the payload drug, and the small molecule linker) and their combination through the chemical conjugation. In principle, the chemical modifications of a mAbs should not impact its binding properties and Fc mediated activities. In the meantime, the multi-step drug conjugation reactions should not impact the efficacy of the drug and stability of the linker. However, in reality, an ADC is prone to many potential chemical degradation pathways and its chemical stability should be extensively evaluated before the deployment into in vivo systems. Scientists from Creative Biolabs provide a comprehensive series of analytical services for ADC chemical stability evaluations. The chemical instability of an ADC can be originated from the antibody portion, the linker, and the conjugation process:
- Chemical instability of the antibody
The chemical modifications in ADC preparation are potential causes for the chemical liability of the antibody. Normally, during the design stage, the highly dynamic chemical hotspots in the mAb are properly evaluated to prevent carry-over instability into the ADC. However, the structural and conformational impacts of the conjugation reaction still present the possibility to expose the buried chemical hotspots in the mAb and rendering them active again. Thus, the stability of the mAb portion will need to be assessed for an ADC.
- Chemical instability of the linkers (payload release)
Preserving the intact conjugate and preventing the release of free toxic payload during circulation and shelf storage is of critical importance in the formulation and development of an ADC. The chemical composition and characteristics of the linker contribute to its stability both in vivo and in long-term storage. Typically, the stability of linkers is monitored directly by HPLC or LC-MS, to evaluate the extent of payload releases. For example, <2% MMAE loss over 10-day buffer incubation was observed using a valine-citrulline peptide linker, while the commonly used hydrazone linker is much less stable biochemically. As a matter of fact, an ADC containing a hydrazine linker has been withdrawn from the market due to the safety issue caused by the instability of this linker.
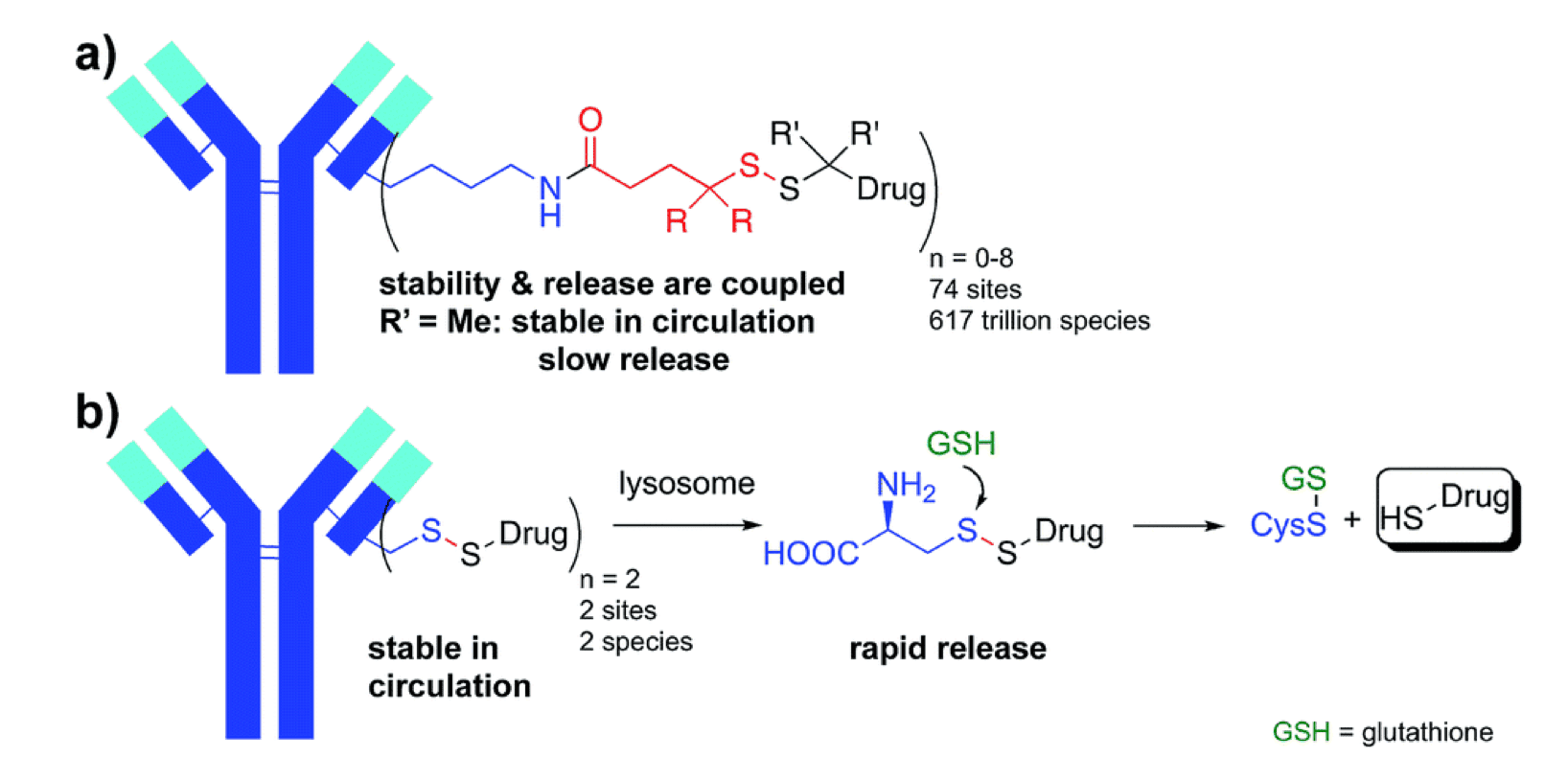 Fig.1 Antibody-small molecule drug conjugates with disulfide linkers.1,2
Fig.1 Antibody-small molecule drug conjugates with disulfide linkers.1,2
- Chemical stability of the conjugation process
The production of an ADC requires multi-step reactions to conjugate the payload drug onto the mAbs. For the first-generation ADCs, the conjugation sites are usually endogenous amino acids including Lys or Cys and some pre-treatment, such as activation and partial reduction, are needed to facilitate the conjugation. Other steps in a conjugation reaction, such as long periods of incubation with different reactive agents, buffer exchange, solvent removal, and product concentration… may all attribute to the chemical instability of the final ADC. For example, the activation step of lysine conjugation introduces the risk of activated products cross-linking. Cysteine-based conjugation requires potential quaternary structure destabilization, as it makes use of partial reduction of disulfide bonds to generate a limited number of free thiols. The next generation of ADC conjugation technologies aims to introduce specific conjugation sites via protein engineering approaches or utilize novel conjugation chemistry to make the process less harsh and ensure the stability of the final ADC products.
Creative Biolabs has established an advanced technological platform with cutting-edge laboratory equipment that enables comprehensive ADC chemical stability analysis. Our equipment includes but not limited to:
- Hydrophobic interaction chromatography (HIC)
- Liquid chromatography-mass spectrometry (LC-MS)
- High performance liquid chromatography (HPLC)
- SDS-PAGE
- ELISA
With our integrated analytical platform and experienced science teams, Creative Biolabs is committed to provide our clients with the most comprehensive analysis services for ADC characterization and we also offer various other services to be your one-stop service station for ADC development. Please contact us for more information and a detailed quote.
References:
- Rosario, GeoffreyáDel. "Decoupling stability and release in disulfide bonds with antibody-small molecule conjugates." Chemical science 8.1 (2017): 366-370.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
