ADC Development Services for Acute Lymphoblastic Leukaemia (ALL) Research
Acute lymphoblastic leukaemia (ALL) is the most common type of childhood leukaemia. Outcomes of conventional chemotherapy for ALL are dismal for patients with relapsed disease, highlighting the need for novel therapies. ADCs represent a kind of powerful and effective anti-cancer agents, which are designed to allow specific targeting of highly potent cytotoxic agents to tumor cells while sparing healthy tissues, and numbers of ADCs are developed actively currently. Creative Biolabs has over a decade of working experience in antibody design and modification, and now we provide comprehensive high-quality ADC preparation services against ALL from design, construction to characterization.
Introduction of ALL
ALL is a cancer that affects the lymphoid line of blood cells. It progresses rapidly and aggressively, characterized by the development of large numbers of immature and abnormal lymphocytes. Symptoms commonly include pale skin color, feeling tired and breathless, repeated infections over a short space of time, frequent bleeding and bruising, high temperature, night sweats, bone and joint pain, swollen lymph nodes (glands) abdominal pain and unexplained weight loss. In most cases, the cause of ALL is unclear. Risk factors often include genetic disorders, exposed to radiation or prior chemotherapy. An abnormal immune response to a common infection is considered as a trigger. The underlying mechanism is associated with multiple genetic mutations leading to rapid cell division. The excessive immature lymphocytes in the bone marrow inhibit the production of new red blood cells, white blood cells, and platelets. Conventional treatments for ALL include chemotherapy, radiation therapy and stem cell transplantation. ADC is a type of novel immunotherapy for this disease.
 Fig.1 Acute lymphoblastic leukaemia (ALL).
Fig.1 Acute lymphoblastic leukaemia (ALL).
ADC Development for ALL
In recent years, remarkable advances have developed in immunotherapy for ALL. Many of these treatments target cell surface antigens found on B lymphoblasts, such as CD22, and CD19. Several ADCs have been designed based on these specific antigens for the targeted treatment of the ALL. CD22 is rapidly internalized upon antibody binding, thus making it an attractive target for delivering conjugated cytotoxic compounds. For instance, Inotuzumab ozogamicin (InO) is an ADC composed of an anti-CD22 monoclonal antibody linked to calicheamicin, a potent cytotoxic compound that induces double-strand DNA breaks and apoptosis. Based on promising clinical trial results, InO was approved by the FDA for the treatment of relapsed and refractory ALL in 2017. Besides, clinical trials are underway evaluating InO in combination with chemotherapy in the relapsed and refractory ALL setting as well as in the frontline setting.
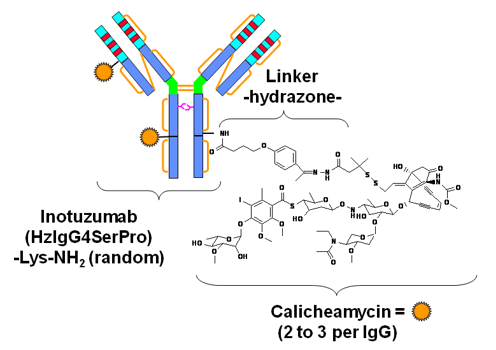 Fig.2 Structures of Inotuzumab ozogamicin (InO).
Fig.2 Structures of Inotuzumab ozogamicin (InO).
Coltuximab ravtansine (SAR3419) is an ADC with a humanized anti-CD19 antibody conjugated to maytansin (an anti-tubulin molecule similar to vincristine) via a cleavable disulfide linker. SAR3419 has entered phase II trial in patients with relapsed/refractory ALL. Denintuzumab mafodotin, also known as SGN-CD19A and SGN-19A, is another ADC consisting of a humanized anti-CD19 antibody conjugated to a microtubule-disrupting agent monomethyl auristatin F (MMAF). Based on the favorable results in a phase I study, patients with relapsed or refractory ALL or lymphoma were treated with SGN-CD19A. This ADC will be warranted to further clinical investigation.
Our ADC development services for ALL include but not limited to:
What Can We Do for You?
ADCs represent an innovative and promising therapeutic method in the cancer treatment. As a well-recognized leader in ADC preparation, Creative Biolabs is committed to providing a full range of ADCs development services targeting ALL. Our professional scientists have extensive experience in antibody production, drug production and bio-conjugation, we are happy to promote your ADC development for innovative cancer treatments. Please feel free to contact us for more details.
For Research Use Only. NOT FOR CLINICAL USE.
Related Sections
ADC Applications in Diseases Research:
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
Contact usUSA
Tel:
Fax:
Email:
Europe
Tel:
Email:
Germany
Tel:
Email:

