- Home
- Applications
- Gastrointestinal Cancer
- Gastrointestinal Malignancy
ADC Development Services for Gastrointestinal Malignancy Research
Gastrointestinal (GI) malignancies are a major global health problem in the world. Although the diagnostic techniques and therapeutic approaches are improved significantly, the survival still remains minor improvement. The development of novel therapeutic agents is critical for the treatment of this cancer. Antibody-drug conjugate (ADC) is a type of novel and effective therapy developed to improve the therapeutic outcome of cytotoxic anti-cancer agents, which allows delivery of the potent drug to the tumor while minimizing exposure of normal tissues. With abundant experience in antibody development, drug production and bio-conjugation, Creative Biolabs offers one-stop-shop development and manufacturing services for your ADCs. Whether you require ADC design, small scale production or commercial scale products, our experts can provide comprehensive services per your request.
Introduction of Gastrointestinal Malignancies
GI malignancies are common and frequently lethal neoplasms, which is a group of cancers that affect the gastrointestinal tract. To date, the specific cause of many types of gastrointestinal malignancies is unknown. Risk factors of the cancer can be divided into different types, including smoking, excessive alcohol consumption, increasing age, diet high in animal fat, diet containing high amounts of salt, chronic pancreatitis and obesity. Symptoms often present abdominal pain, tenderness, or discomfort, rectal bleeding or blood in stool, bloating, loss of appetite, nausea/vomiting, unintentional weight loss and fatigue. Currently, chemotherapy, radiation therapy and surgery are the most common treatment approaches for GI malignancies.
ADC Development for Gastrointestinal Malignancies
ADCs represent a fast-growing new class of anticancer therapeutics, which are designed to selectively carry a potent cytotoxic drug to tumors, while showing limited systemic toxicity to healthy tissues. In recent years, several ADCs are investigated in various stages of clinical development for the treatment of gastrointestinal malignancies to improve patient survival.
- TAK-264 is a novel ADC consisting of a fully human IgG1 monoclonal anti-GCC (transmembrane cell surface receptor guanylyl cyclase C) antibody conjugated to MMAE via a protease-cleavable linker. Following binding to GCC, TAK-264 can be internalized and the MMAE is released, resulting in cell-cycle arrest and apoptosis. In the phase I study of TAK-264, results showed a manageable safety profile at the maximum tolerated dose, a positive antitumor activity and clinical benefit in patients with gastrointestinal malignancies.
- TAK-164 is an ADC composed of an anti-GCC human IgG1 monoclonal antibody linked to indolino-benzodiazepine DNA alkylator DGN549 with a peptide linker. Results demonstrated that TAK-164 can efficiently bind to antigen-expressing cells and be internalized causing obvious cytotoxicity. Besides, after a single administration of TAK-164 in animal models with GCC-positive xenografts, results showed that the ADC has a low plasma clearance. Besides, antitumor activity study suggested that TAK-164 possesses durable antitumor activity. Thus, these promising preclinical data warrant the advancement of this ADC to clinical evaluation.
- Cantuzumab mertansine is another novel ADC consisting of an anti-CanAg antibody (huC242) and a potent maytansinoid antimicrotubule agent DM1. This ADC is under Phase I study, evidence revealed that this ADC has broad activity against a range of CanAg-positive human tumor. Other ADCs targeting the gastrointestinal malignancies such as the PF-06263507 and SAR408701 also are investigated actively.
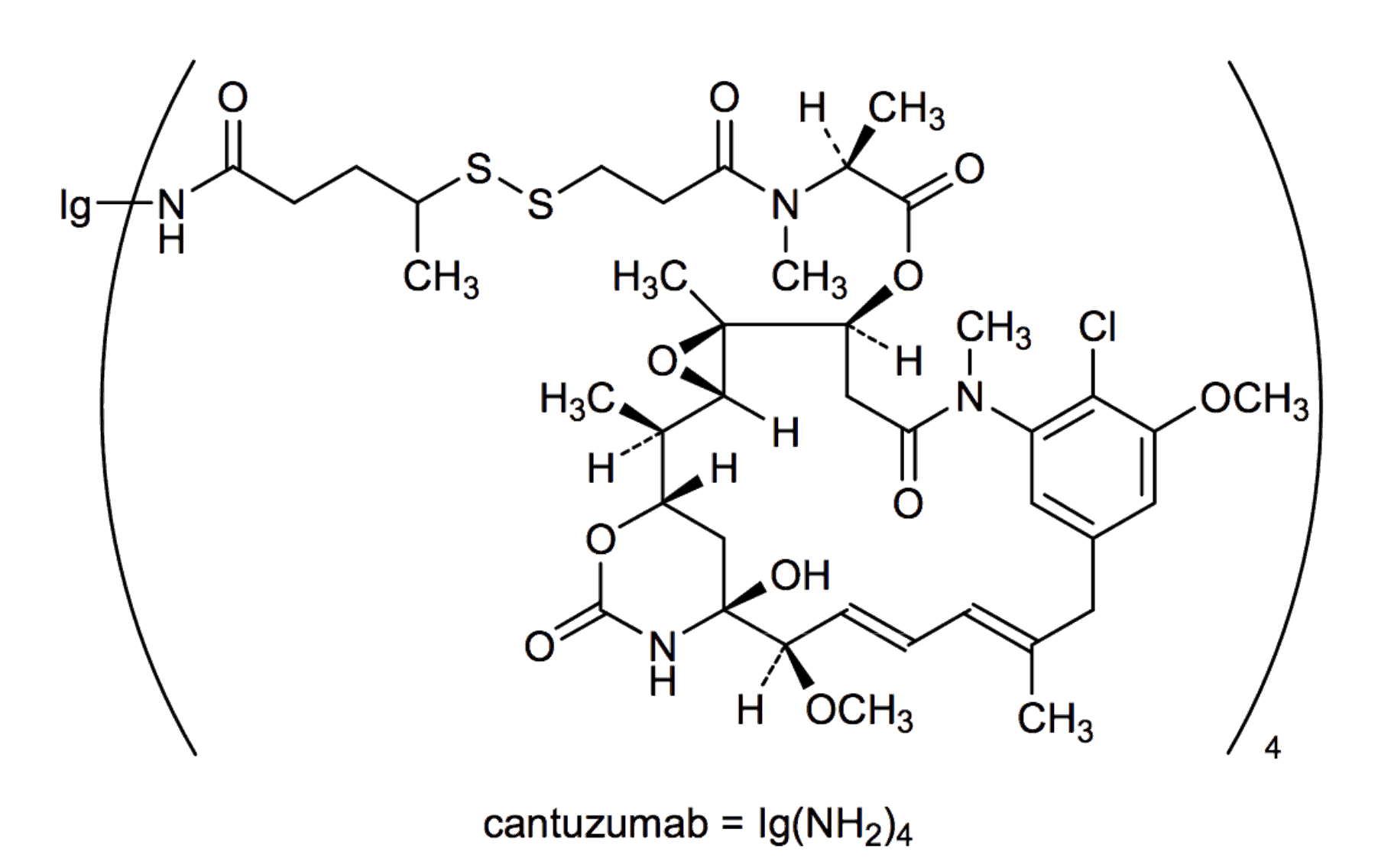 Fig.2 The structure of cantuzumab mertansine.
Fig.2 The structure of cantuzumab mertansine.
What Can We Do for You?
At Creative Biolabs, targets of ADC development for gastrointestinal malignancies include:
Creative Biolabs is a leading service provider in the field of ADCs design and preparation worldwide. We provide a fully integrated ADC development services against gastrointestinal malignancies. Our services include high affinity antibody development, high-quality drug production and efficient antibody-drug conjugation. Please contact us for more information if you are interested in our services.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
