ADC Development Services Targeting CD22
Antibody-drug conjugates (ADCs) combine the targeted binding specificity of monoclonal antibodies and the cytotoxic potency of small molecule chemotherapy agents via conjugation with chemical linkers. Several approved ADCs products and a large number of candidates in clinical and pre-clinical development highlight that ADCs are emerging as one of the most promising biologics in anti-cancer treatment. Based on years of extensive experience in antibody development and bioconjugation, Creative Biolabs offers a wide range of high-quality ADCs development services against CD22 marker, from antibody production to drug-antibody conjugation.
Introduction of CD22
CD22 is a member of the SIGLEC family of lectins, which is a single chain transmembrane protein. It can bind sialic acid through an N-terminal immunoglobulin (Ig) domain and involved in regulating the expression of the B cell receptor (BCR) IgM signaling as an adhesion receptor and a signaling molecule. It is commonly produced at the earliest stage of B-cell differentiation in cytoplasmic, and TdT-positive precursor B-cells. CD22 often is highly expressed in some immunoglobulin signaling related diseases, such as the B-ALL, chronic lymphoid leukemias, and B-cell lymphomas. However, CD22 is absent in T-cells and their malignant counterparts. Besides, this protein also plays a role in inhibiting the BCR signaling as an inhibitory receptor. In mice, it functions in the B cell trafficking to Peyer's patches.
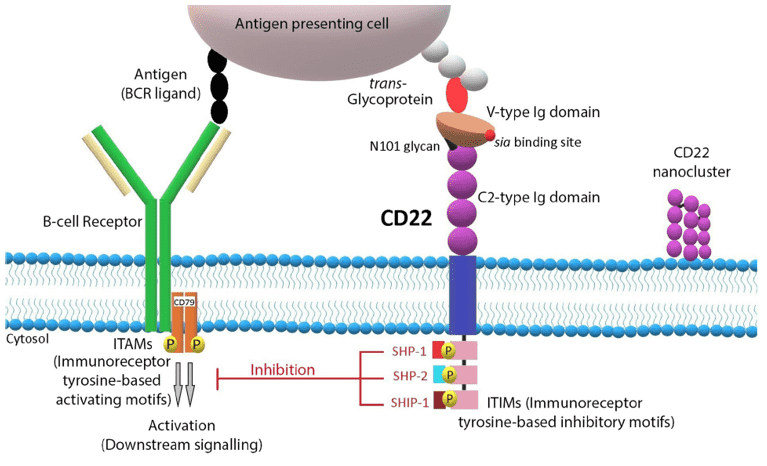 Fig.1 CD22 structure and signaling pathway.1,2
Fig.1 CD22 structure and signaling pathway.1,2
Anti-CD22 ADC for non-Hodgkin Lymphoma (NHL)
NHL is the most common type of lymphoma, which is cancer originating from the lymphatic system. Although traditional treatments such as chemotherapy, radiation therapy, and surgery show advances in the clinical outcomes of patients with NHL, indolent B-cell malignancies still remain incurable especially patients with aggressive NHL. Thus, new therapies need to be explored for better treatment results.
Pinatuzumab vedotin (also known as RG7593 or DCDT2980S), an ADC for the NHL, consists of a humanized anti-CD22 monoclonal IgG1 antibody linked to the potent microtubule-disrupting agent, monomethyl auristatin E (MMAE), via a protease cleavable linker, maleimidocaproyl-valine-citrulline-p-aminobenzoyloxycarbonyl (MC-vc-PAB). Preclinical trial results showed that pinatuzumab vedotin was capable of inducing complete tumor regression in xenograft models of NHL and can be more effective than rituximab plus combination chemotherapy. Besides, phase I trial results in patients with relapsed or refractory B-Cell non-Hodgkin’s lymphoma and chronic lymphocytic leukemia demonstrated that the pinatuzumab vedotin has an efficacy, safety, and pharmacokinetics profile to support the potential treatment of NHL.
Anti-CD22 ADC for Acute Lymphoblastic Leukemia (ALL)
ALL is a kind of cancer that affects the lymphoid line of blood cells. Inotuzumab ozogamicin (InO) is an ADC composed of anti-CD22 humanized IgG4 monoclonal antibody conjugated to calicheamicin via an acid-labile linker. Calicheamicin is a potent cytotoxic drug that binds DNA in the minor groove and induces double-strand DNA breaks and cell apoptosis. Once the ADC bound to the CD22, the endocytosis process leads to the release of the payload in the acidic lysosomal environment to kill the tumor cells. Clinical trials have shown superior survival with the ADC over conventional approaches in the first- or second-line salvage therapy for relapsed B-ALL. Notably, Inotuzumab ozogamicin was approved for the treatment of relapsed and refractory B-ALL in 2017. What’s more, the combination of InO with chemotherapy is underway evaluated actively in clinical trials for the relapsed and refractory ALL.
Anti-CD22 ADC for Chronic Lymphocytic Leukemia (CLL)
CLL is a cancer of the blood and bone marrow, due to bone marrow produces excess lymphocytes. Inotuzumab ozogamicin (InO) ADC also presents positive treatment outcomes for the CLL in clinical trials.
What Can We Do for You?
Creative Biolabs has focused on the ADCs development and related services in the last years, including antibody production, drug/linker synthesis and bio-conjugation. We have professional team and advanced laboratory and we can provide a comprehensive set of ADCs development services targeting the CD22. If you are interested in our service, please do not hesitate to contact us for more details. Our ADCs development services including:
- ADC Antibody Screening
- DrugLnk™ Custom Synthesis
- Antibody Design and Conjugation
- ADC in vitro Analysis
- ADC in vivo Analysis
References
- Aujla A, Aujla R, Liu D. Inotuzumab ozogamicin in clinical development for acute lymphoblastic leukemia and non-Hodgkin lymphoma[J]. Biomarker Research, 2019, 7(1): 9.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
