ADC Development Services Targeting GCC
Recently, Antibody-drug conjugate (ADC) has emerged as a novel and effective agent in cancer therapeutics, which can achieve targeted delivery of the drug to cancer cells by taking advantage of the specificity of monoclonal antibodies. Creative Biolabs has extensive experience in providing drug-linker synthesis, antibody-drug conjugate and ADC characterization services. We offer one-stop ADCs construction services targeting GCC for clients all over the world.
Introduction of GCC
GCC (transmembrane cell surface receptor guanylyl cyclase C) antigen is overexpressed on gastrointestinal malignancies cell and has become a promising target for ADCs development. GCC is an enzyme in humans discovered in the 1970s and is encoded by the GUCY2C gene. It is an intestinal receptor that can recognize the exogenous diarrhoeagenic bacterial heat-stable enterotoxins (STs). GCC and its associated signaling pathways play critical roles in the maintenance of normal physiological functioning of the GI tract. The activation of the GCC receptor can regulate the homeostasis of fluid and ion, maintain the intestinal barrier, inhibit inflammation and modulate the visceral pain signaling and tumourigenesis. Disorder of the GCC signaling pathways will cause a variety of GI diseases, such as the inflammatory bowel diseases (IBDs), colorectal cancers (CRCs), constipation and diarrhea. Currently, GCC receptor and its ligands are considered as attractive targets for therapy in diverse gastrointestinal diseases, including gastrointestinal malignancies, functional gastrointestinal disorders (FGIDs) and IBDs.
ADC Development Targeting GCC
ADCs are a type of antibody-directed cancer therapy that can improve the therapeutic efficacy and decrease systemic toxicity. GCC as a potential target has been used in the ADCs construction for some GI associated disorders treatment. For example, MLN0264 (TAK-264) is a novel ADC targeting the GCC investigated in patients with gastrointestinal (GI) malignancies. TAK-264 is composed of a specific anti-GCC antibody conjugated to the potent anti-microtubule agent monomethyl auristatin E (MMAE) via a protease-cleavable linker. The ADC can specifically combine with GCC, and then be internalized and transported to lysosomes to release drugs, leading to cell cycle arrest and apoptosis. TAK-264 showed significant anti-cancer activity in clinical studies in patients with gastric, gastroesophageal, and pancreatic cancer.
TAK-164 is another ADC targeting the GCC antigen, which consists of an anti-GCC human IgG1 monoclonal antibody and the indolino-benzodiazepine DNA alkylator DGN549 via a peptide linker. During the preclinical trials of the ADC, results showed that TAK-164 can be internalized obviously and then resulting in significant cancer cell cytotoxicity. Besides, research also demonstrated that this ADC presents a long-term half-life in plasma and durable antitumor activity. TAK-164 will be warranted to clinical investigation subsequently.
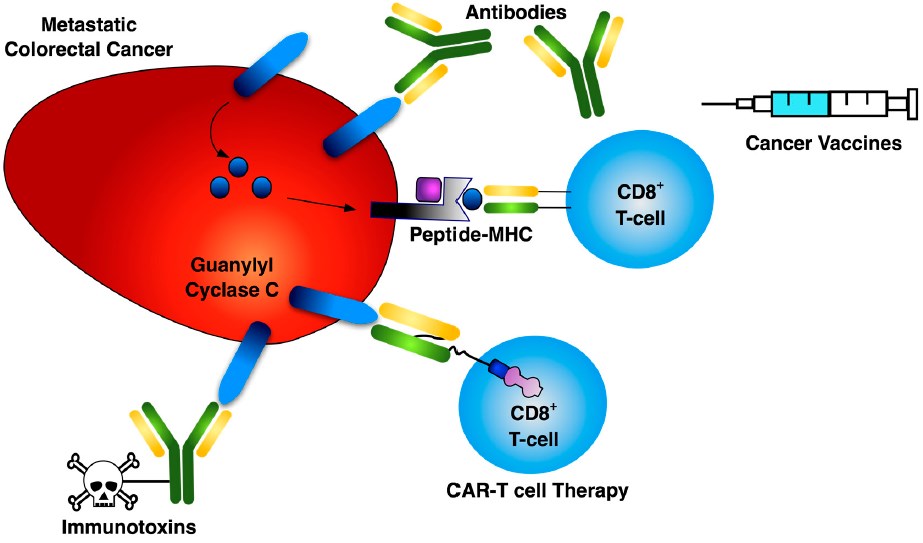 Fig.1 Three primary modalities for GUCY2C-directed cancer immunotherapy.1,2
Fig.1 Three primary modalities for GUCY2C-directed cancer immunotherapy.1,2
What Can We Do for You?
ADCs offer a promising therapeutic modality aimed at enhancing the target-directed cancer treatment. Creative Biolabs, a leading service provider in the area of ADCs, have completed a large number of ADC-related projects. Currently, Creative Biolabs provides a comprehensive ADCs design, construction and analysis services. If you are interested in our services, please contact us for more information.
Our featured ADCs development services include:
- ADC Antibody Screening
- DrugLnk™ Custom Synthesis
- Antibody Design and Conjugation
- ADC in vitro Analysis
- ADC in vivo Analysis
References
- Baybutt, Trevor R., Allison A. Aka, and Adam E. Snook. "The heat-stable enterotoxin receptor, guanylyl cyclase C, as a pharmacological target in colorectal cancer immunotherapy: A bench-to-bedside current report." Toxins 9.9 (2017): 282.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
