- Home
- ADC Development
- ADC In Vitro Analysis
- ADC In Vitro Efficacy Evaluation
- ADC Fc Cytotoxicity Evaluation
ADC Fc Cytotoxicity Evaluation Service
As an industrial leader in therapeutic antibody development, Creative biolabs has extensive experience in antibody effector activity characterization. We employ our comprehensive in vitro efficacy analysis platform to give a full evaluation of the ADC in vitro efficacy contributed by the effector activities of the antibody portion.
Antibody-drug conjugates (ADCs) are a unique class of novel anti-tumor agents produced by the conjugation of highly cytotoxic drug payloads with tumor specific monoclonal antibodies via elaborate chemical linkers. They are usually defined as the “next generation” targeted medicine. By design, an ADC functions through the internalization via receptor mediated endocytosis upon antigen binding and deliver the cytotoxic drugs to their corresponding subcellular targets. The major cytotoxicity, or “efficacy”, of an ADC resides in the toxic “war-head” it carries. However, an ideal ADC should also harvest the “endogenous” toxicity of the antibody Fc region to recruit effector cells/molecules and achieve a “secondary” tumor killing efficacy via various mechanisms such as antibody dependent cellular cytotoxicity (ADCC), complement dependent cytotoxicity (CDC), antibody dependent cellular phagocytosis (ADCP)…. What's more, the antibody portion of an ADC can also lead to the uptake of tumor associated antigens by antigen-presenting cells and establish T cell memory effect.
Creative biolabs provides various antibody dependent cytotoxicity assays to fully evaluate the effector activities of any customized ADCs. Our assays mainly include:
Antibody dependent cellular cytotoxicity (ADCC)
- Mechanism and process:
- ADC binding to targeted cancer cells.
- ADC Fc recognition by Fc receptors expressed on immune cells (natural killer cells, macrophages, dendritic cells etc.).
- Recruited immune cells activation and accomplish tumor cell killing via the releasing of perforin and granzymes or expression of tumor necrosis factor (TNF) family proteins.
- Detection methods: lactate dehydrogenase (LDH) release assay or chromium (51Cr) release assay.
- Commonly used effector cells: peripheral blood mononuclear cells (PBMCs), isolated natural killer (NK) cells, or macrophages.
Complement dependent cytotoxicity (CDC) or complement dependent cellular cytotoxicity (CDCC)
- Mechanism and process:
- ADC binding to targeted cancer cells.
- Complement protein C1q binding to the Fc portion on the ADC to activate complement system.
- Tumor cell killing accomplished via the formation of membrane attack complex to lyse tumor cells or the recruitment and activation of certain immune effector cells (macrophages, nature killer cells etc.) for target elimination.
- Detection methods: fluorescence labeling and flow cytometry analysis.
Antibody dependent cellular phagocytosis (ADCP)
- Mechanism and process:
- ADC binding to targeted cancer cells.
- ADC Fc recognition by Fc receptors expressed on macrophages or other phagocytic cells.
- Tumor cell killing accomplished by opsonisation and phagocytosis.
- Detection methods: fluorescence labeling and flow cytometry analysis
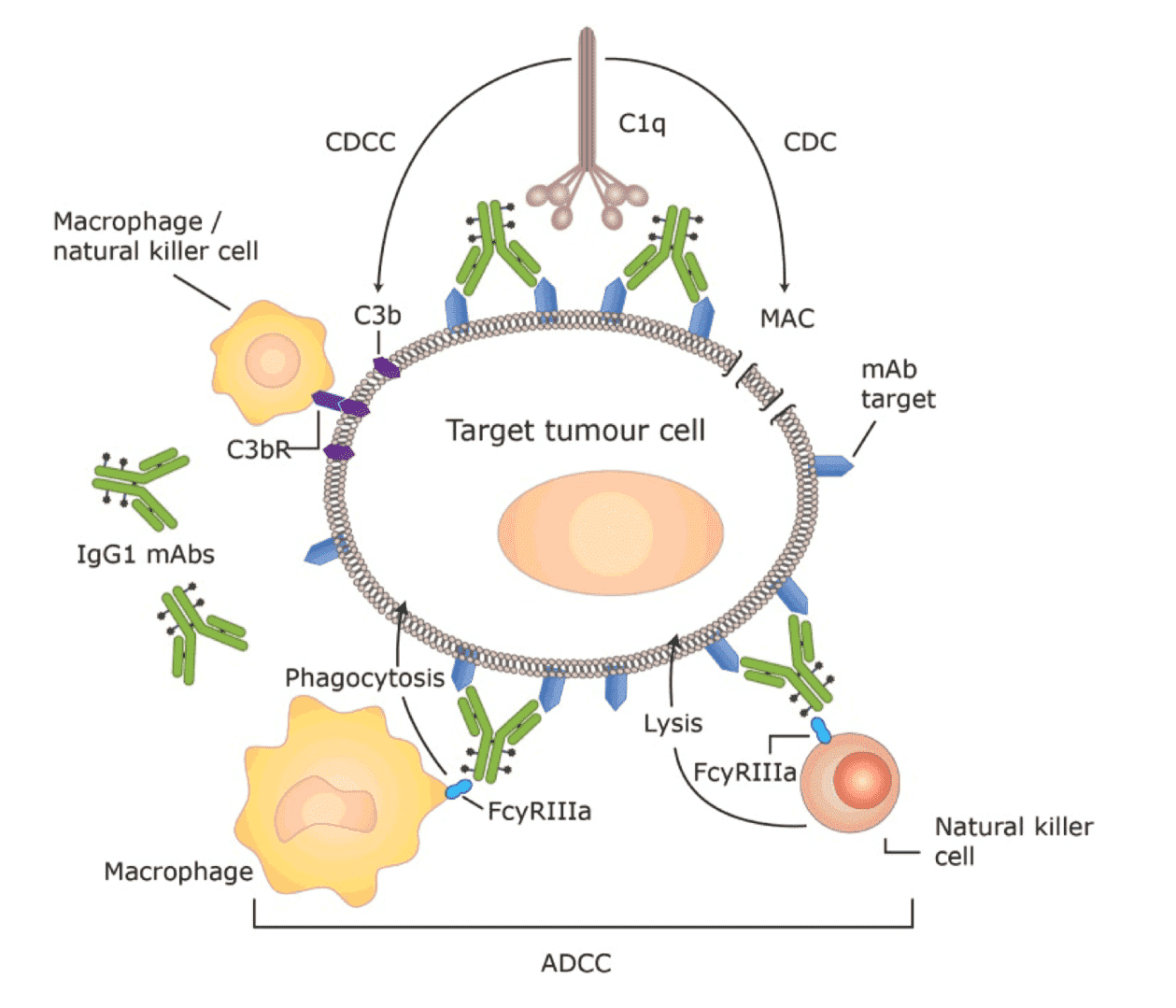 Fig.1 Fc effector activities, a secondary cell killing mechanism that contributes to ADC efficacy.1,2
Fig.1 Fc effector activities, a secondary cell killing mechanism that contributes to ADC efficacy.1,2
With years of accumulative work in antibody discovery, pharmaceutical chemistry synthesis, and therapeutics analysis, Creative biolabs has established an advanced analytical platform to provide our global customers with integrated antibody-drug conjugates characterization services in a timely and cost efficient manner. Please contact us for more information and a detailed quote.
References:
- Peters, Christina, and Stuart Brown. "Antibody–drug conjugates as novel anti-cancer chemotherapeutics." Bioscience reports 35.4 (2015): e00225.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
