- Home
- ADC Development
- ADC In Vivo Analysis
- ADC In Vivo Efficacy Evaluation
ADC In Vivo Efficacy Evaluation Services
Antibody-drug conjugates (ADCs) are under active developments as a class of highly potent biopharmaceutical agents for oncological and hematological disorder treatments. In recent years, the approval of several ADCs by FDA has inspired great investments and more advanced developments of antibody bio-conjugate therapeutics.
One important pharmacological parameter of an ADC is the in vivo efficacy that directly reflects its potency and influences clinical trial designs. As an elaborately conjugated entity, the effector activity residues in the Fc portion of the monoclonal antibody and the carried cytotoxin both contribute to the in vivo efficacy of an ADC, making the assessment more complicated. As a recognized leader in cancer immune therapy, Creative Biolabs has been supporting anti-cancer studies for decades and we fully understand and appreciate the intricacy in ADC efficacy both in vitro and in vivo. We are committed to provide the scientific community with well-established tumor models for ADC in vivo efficacy assessment. All of our animals are maintained in a clean and feed-enriched environment and related experiments are followed Institutional Animal Care and Use Committee (IACUC) approved protocols.
Animal Resources
Creative Biolabs possesses various laboratory animals including rodents (mice, rat, rabbit) and non-rodents (beagle dog and non-human primate) to support versatile demands for pre-clinical trials of new drugs. Our animal center is comprised of an enclosed animal housing facility, an animal care facility, and numerous cutting-edge equipment for animal processing. Our technical support team is led by Ph.D. level professionals with extensive knowledge and experience in all aspects of animal researches.
Animal Oncological Models
Creative Biolabs offers a diverse selections of animal oncological models for ADC efficacy assessments. The most widely used animal oncological models are xenograft models developed by transplanting human malignant cells or tissues into immunocompromised mice or other rodents. Malignant cells are usually amplified to a large number and injected into animals subcutaneously, intravenously, or orthotopically to allow tumor formation and growth.
Creative Biolabs maintains a wide variety of human cancer cell lines for xenograft tumor model development:
| Condition | Target | Cell Line |
| Acute myeloid leukemia | CD33 | U937, THP-1, MV-4-11, TF-1a, ML-2, HL-60, L1210, K562 |
| Pancreatic cancer | CD74, CD227, nectin-4 | Capan-1, Capan-2, CFPAC-1, HPAF-II, MIAPaCa-2, PANC-1 |
| Breast cancer | CD174, GPNMB, CRIPTO, nectin-4, LIV1A, HER2 | Bcap-37, BT-474, ZR-751, ZR-75-30, MKN-28, NCL-N87 |
| Ovarian cancer | MUC16, TIM-1, mesothelin | ES-2, HO-8910PM, PA-1, SK-OV-3 |
| Melanoma | GD2, GPNMB, ED-B, PMEL 17, endothelin B receptor | A375, B16, A2058, C32, SK-MEL-30 |
| Prostate | PSMA, STEAP-1, TENB2 | 22Rv1, CL-1, DU145, PC-3 |
| Renal | CAIX, TIM-1 | OS-RC-2, 768-O, ACHN |
| Mesothelioma | Mesothelin | |
| Colorectal cancer | CD74, CD174, CD227, CD326, CRIPTO, FAP, ED-B | COLO201, COLO205, COLO320DM, CW-2, HCT-8, HCT-15, HCT116. SW480, SW620 |
| Lung | CD56, CD326, CRIPTO, FAP, mesothelin, GD2, 5T4 | NCL-H526, NCL-H1688, NCL-H69, NCL-H146, NCL-H209, NCL-H446, NCL-H226 |
| Multiple myeloma | CD56, CD74, CD138, endothelin B receptor | KMS-26, RPMI-8226, KMS-11 |
Subcutaneous xenograft model is mostly used for pre-clinical tests. Creative Biolabs also provides genetically engineered mouse models (GEMM). In this model, tumor growth is triggered by conditional activation or inactivation of the tumor suppressive oncogene. Successful tumor formation in GEMM is selected by palpation or radiograph.
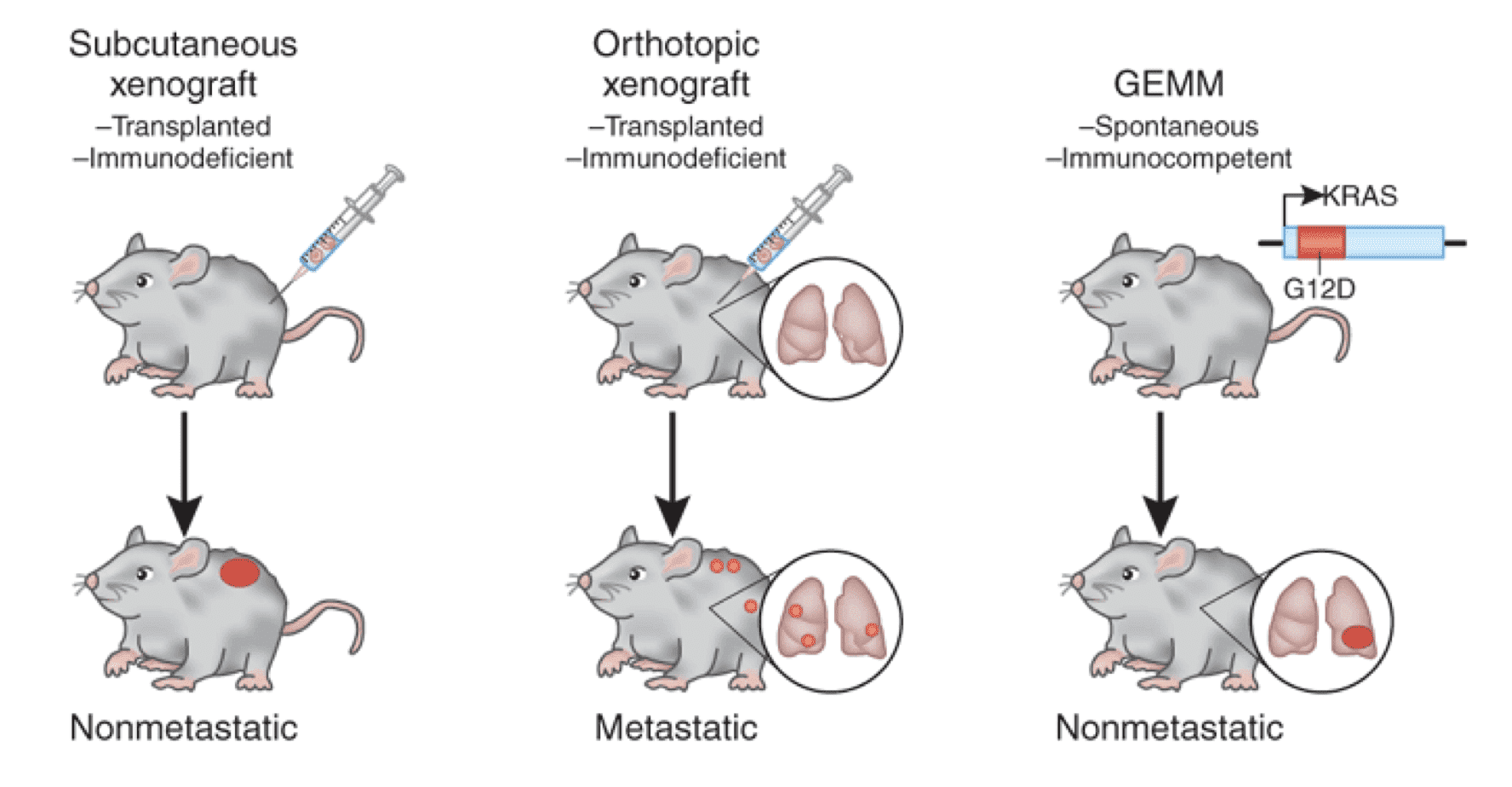 Development processes of xenograft tumor and GEM models in mice. Xenograft model: Subcutaneously injection of tumor cell line or directly implantation of patient-derived tissue into immunedeficient mouse. GEM model: mouse genetic profile is altered and leads to carcinomatous condition and react therapeutic treatments.
Development processes of xenograft tumor and GEM models in mice. Xenograft model: Subcutaneously injection of tumor cell line or directly implantation of patient-derived tissue into immunedeficient mouse. GEM model: mouse genetic profile is altered and leads to carcinomatous condition and react therapeutic treatments.
Please follow the links to find more specific information about our animal model establishment and ADC in vivo efficacy assessment for Solid Tumor and Hematological Malignancy.
Clinical Observation
After ADC administration in the model animals, we perform systematic clinical observations to assess the direct effect of ADCs. The clinical assessment, including cage-side observation and clinical check, are conduct by experienced technicians while physical parameters, such as body weight, food consumption, and physiological changes of the tested animals will be recorded and reported in detail.
Pathological Analysis
Creative Biolabs provides cutting-edge techniques and expertise in pathological evaluation. Impacts of ADC treatments in tumor cells and their effect on normal cells or tissues are visualized and analyzed in organ/tissue examinations using methods such as macroscopic assessment and immunohistochemistry.
With our technical expertise and commitment to serve the scientific community, Creative Biolabs is dedicated in providing our clients with high-quality services for the assessment of the in vivo efficacy and other in vitro or in vivo parameters of an ADC. Please contact us for more information and a detailed quote.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
