- Home
- UTC Development
- Antibody-Enzyme Conjugate Development
- Antibody-directed Toxic Enzyme Fusion Protein Development
- Antibody-Onconase & RapLR1 Fusion Protein
Antibody-Onconase and RapLR1 Fusion Protein Development Service
With years of experience, Creative Biolabs offers customized antibody-onconase & RapLR1 fusion protein with first-class technology and platform. Our optimized fusion expression strategy will promote the development of innovative cancer treatments.
Onconase & RapLR1
Onconase is an amphibian ribonuclease found in oocytes of the leopard frog. It is a member of the pancreatic ribonuclease (RNase A) protein superfamily which targets RNA substrates with a sequence preference for uracil and guanine nucleotides. It has been extensively studied for its potent antitumor properties, as it has a naturally high selectivity for cancer cells. The clinical trials of onconase have shown that it has low immunogenicity and very little nonspecific toxicity. The mechanism of onconase to perform tumor cytotoxicity selectively is via cleaving siRNA molecules to interfere with the RNA pathway. As a result, the NF-кB pathway can interfere. At present, onconase is in Phase I clinical study for the treatment of anogenital warts. Rana pipens liver RNase1 (RapLR1), a close homolog of onconase, offers an advantage over other amphibian ribonucleases because it retains its ribonucleolytic and cytotoxic activity without post-translational modifications of the N-terminus (the active site).
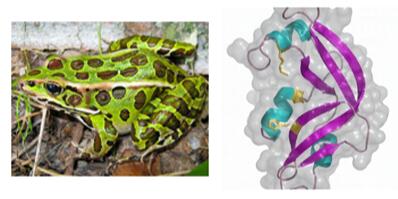 Fig.1 Mechanism of action. In cancer cells, onconase-induced intracellular RNA đegradation triggers several biological effects simultaneously including the induction of the "intrinsic" (mitochondria) pathway of apoptosis and modulation of the expression of several key cell cycleregulatory factors.
Fig.1 Mechanism of action. In cancer cells, onconase-induced intracellular RNA đegradation triggers several biological effects simultaneously including the induction of the "intrinsic" (mitochondria) pathway of apoptosis and modulation of the expression of several key cell cycleregulatory factors.
Antibody-onconase & RapLR1 Fusion Protein
In early studies, antibody-onconase chemical conjugates were used to treat B-cell lymphoma xenografts effectively in immunodeficient mice, demonstrating potency comparable to immunotoxins without mediating nonspecific toxicity. Second-generation (fusion protein) immunoRNases have proven to be promising fusion proteins for targeting various malignancies.
With our advanced technology platforms and experienced scientists, Creative Biolabs is committed to helping you develop antibody-onconase & RapLR1 fusion protein in a timely and cost-effective manner. Our high-quality services and products will contribute greatly to the success of your projects. Please feel free to contact us for more information if you are interested in the antibody-onconase & RapLR1 conjugate.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
