- Home
- UTC Development
- Bispecific ADC Development
- Fast-internalizing Receptor based Bispecific ADC Development
- CD22 based Bispecific ADC Development
CD22 based Bispecific ADC Development Service
Antibody-drug conjugates (ADCs) targeting CD22 can induce fast internalize upon binding to the target, which is considered essential for ADC activity, and have demonstrated preclinical antitumor activity. A bispecific antibody targeting both tumor marker and fast-internalizing receptor could have superior properties to either parental monoclonal antibody (mAb) alone or even a combination of both. With years of experience in ADC preparation, characterization, and our optimized bispecific antibody preparation platform, Creative Biolabs is capable of providing CD22-based bispecific ADC development services as well as ADC in vivo and in vitro evaluation for our global clients.
The Overview of CD22
CD22, a 135 kDa type I transmembrane sialoglycoprotein, is a B-cell lineage-restricted cell surface glycoprotein. It is expressed on the majority of B-cell hematologic malignancies including non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia (CLL), acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), follicular lymphoma (FL), marginal zone lymphoma (MZL), mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), and small lymphocytic lymphoma (SLL). Importantly, CD22 is not expressed on hematopoietic stem cells, memory B cells, or other normal nonhematopoietic tissues. Its expression pattern and rapid internalization kinetics make it a promising target for ADC-mediated treatment of B-cell malignancies and it has been tested in several clinical trials against NHL and ALL.
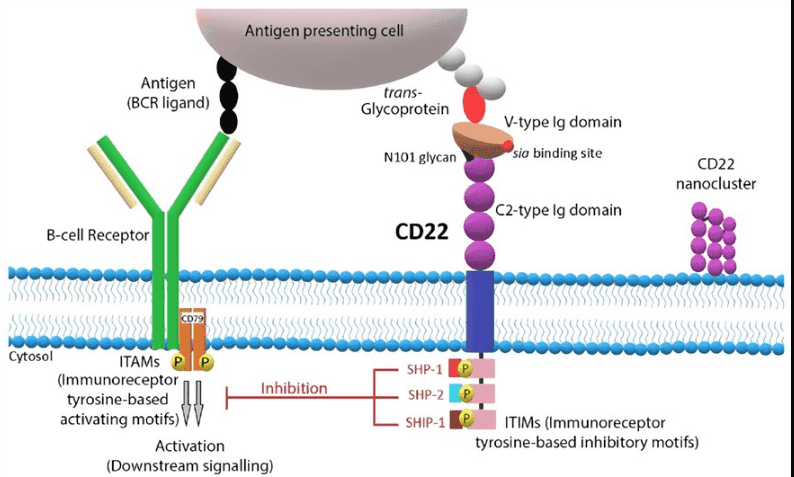 Fig.1 CD22 structure and signaling pathway.1,2
Fig.1 CD22 structure and signaling pathway.1,2
Antibody-based Therapeutics Targeting CD22
The lineage specificity of CD22 makes it an attractive target for antibody-based therapeutics. Multiple therapeutic antibodies targeting CD22 are currently in preclinical and clinical development. For instance, Epratuzumab, a mAb targeting CD22, relies on antibody-dependent cellular cytotoxicity (ADCC) for antitumor activity. It has been tested clinically and has an acceptable safety profile as well as single-agent activity in patients with DLBCL and indolent NHL (iNHL). Besides, anti-CD3/CD22 bispecific antibody, anti-CD19/CD22 bispecific antibody, and anti-CD20/CD22 bispecific antibody have already been developed with favorable pharmacokinetics and in vivo stability.
Based on the existing therapeutic antibodies, many CD22-targeting ADCs have emerged, such as Pinatuzumab vedotin, CAT-02-106, DCDT2980S, and so forth. CAT-02-106 displayed good biophysical characteristics, and mediated efficacy ranging from significant tumor growth delay to complete response in vivo against NHL xenograft models. DCDT2980S has an efficacy, safety, and pharmacokinetics profile that supports potential treatment of NHL. Together, these data supported the clinical feasibility of a CD22-directed antibody and ADCs for the treatment of B-cell malignancies.
What Can We Do for You?
Results from many studies substantiate CD22 as a clinically validated ADC target and open up the possibility of developing bispecific ADCs targeting CD22, ultimately leading to ADC combinations to deliver payload agents more effectively than bispecific antibody alone. Creative Biolabs has demonstrated that selecting the appropriate combination of affinity optimized CD3/CD22, CD19/CD22, and CD20/CD22 ADC variants could lead to higher selectivity for tumor versus normal tissue, which could broaden the therapeutic index.
Creative Biolabs has been involved in the development of ADCs for many years. Now we provide the best CD22-based bispecific ADC construction services with our mature and comprehensive ADC and BsAb platforms. Our one-stop ADCs development services include ADC Antibody Screening, DrugLnk™ Custom Synthesis, Antibody Design and Conjugation, ADC in vitro Analysis, and ADC in vivo Analysis. For more information, please do not hesitate to contact us.
Reference
- Aujla, Amandeep, Ravijot Aujla, and Delong Liu. "Inotuzumab ozogamicin in clinical development for acute lymphoblastic leukemia and non-Hodgkin lymphoma." Biomarker Research 7.1 (2019): 9.
- Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
