Glycan in Immunology and Rheumatology
Currently, as a leader in the field of antibody research and development, Creative Biolabs is committed to providing the most comprehensive anti-glycan antibody development services for our global clients. Creative Biolabs has accumulated abundant experience in the successful completion of many antibody-related projects with the best quality and the most competitive prices. We believe our services and products will suit the exact requirements of our customers.
Introduction
Since the discovery of altered IgG glycosylation in patients with rheumatoid arthritis, there has been increasing evidence supporting the role of glycans in the pathophysiology of autoimmunity. For example, it is now well established that the type of glycan present at residue Asn-180 of IgG1 can enhance the effector function of the antibody, with some glycans being pro-inflammatory while others harboring anti-inflammatory properties. Actually, some autoimmune diseases are strongly related to a particular Ig class or subclass. Thus, in antibody-mediated autoimmunity, the glycan/Ig isotype combination will determine the physical nature of the attack. Importantly, the glycosylation patterns of immunologically relevant cells and serum proteins are associated with gene expression profiles, environmental factors, and the age of the glycans are equally critical. In general, each autoimmune disease will have a unique glycan signature characterized by the site-specific relative abundances of individual glycan structures present on immune cells and serum proteins, especially the site-specific glycosylation patterns of the different Ig classes and subclasses.
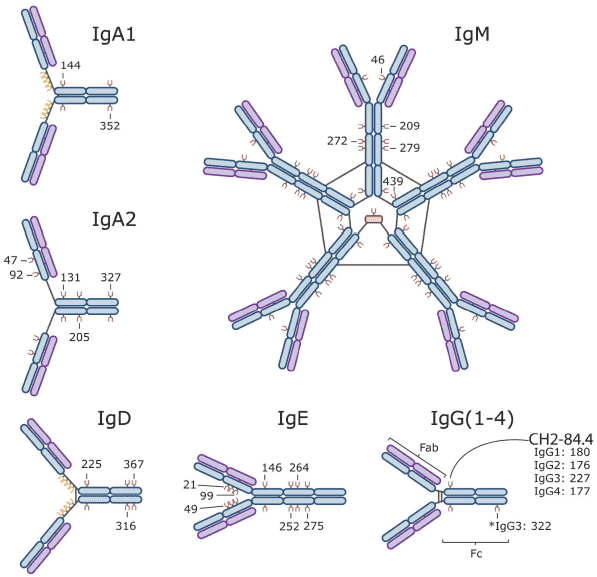 Fig.1 Immunoglobulin Isotypes and their sites of glycosylation. (Maverakis, 2015)
Fig.1 Immunoglobulin Isotypes and their sites of glycosylation. (Maverakis, 2015)
The Role of Glycans in Immunology and Rheumatology
IgG immunoglobulins commonly are N-glycosylated in the constant (CH2 or Fc) region, which possesses several unusual properties. The crystal structure of the protein shows that they are immobilized by glycan-protein interactions. The IgG complex-type biantennary N-glycans are rarely fully sialylated, and instead have one or two terminal β-linked galactose residues (termed G1 and G2, respectively). IgG of patients with rheumatoid arthritis (RA) has less galactose or none at all (termed G0). The severity of this inflammatory disease tends to be associated with the level of galactosylation. One function of the Fc N-glycans is to maintain the conformation of the Fc domains as well as the hinge regions.
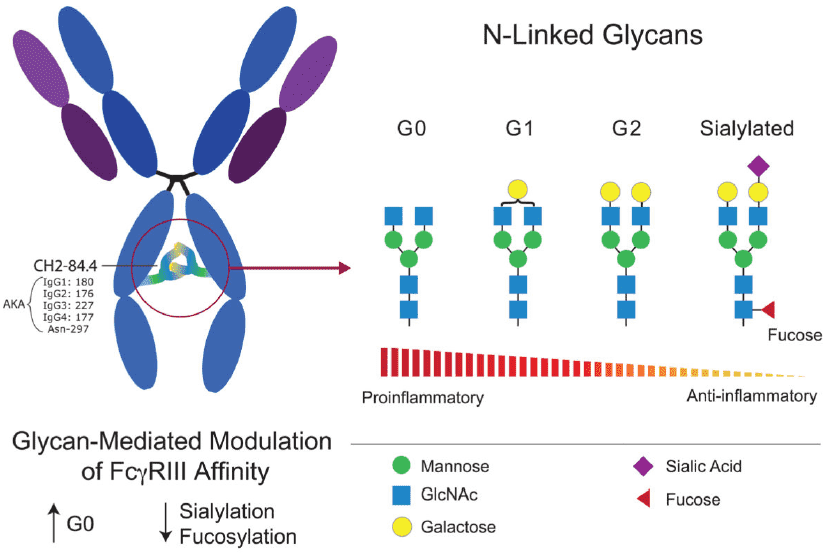 Fig.2 IgG Glycoforms and their inflammatory properties. (Maverakis, 2015)
Fig.2 IgG Glycoforms and their inflammatory properties. (Maverakis, 2015)
The previous study showed that the G0 N-glycans have increased mobility, which is attributed to the loss of interactions between the glycan and the Fc protein surface. Thus, it is considered that regions of the protein surface normally covered by the glycan are exposed in RA. Subsequent studies found that the circulating mannose-binding protein can recognize the mobile G0 N-glycan and activate complement directly. Another possibility is that the altered glycosylation influences interactions with Fc receptors. The glycan changes are also seen to a lesser degree in osteoarthritis, a form of chronic degenerative arthritis with different pathogenesis.
What Can We Do for You?
Equipped with multiple leading technologies and abundance expertise, Creative Biolabs is confident in delivering satisfactory anti-glycan antibodies for immune diseases diagnosis such as the RA with our first-class professional service. We provide quite flexible options about expression, solubilization/reconstitution, and purification as well as characterization (such as binding assays, functional assays). If you are interested in our service, please do not hesitate to contact us for more details.
Reference:
- Maverakis, E.; et al. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review. Journal of autoimmunity. 2015, 57: 1-13.
