Today, when talking about cancer color change, pancreatic cancer can be said to be a “tough role” in cancer. It is highly malignant and ranks seventh in the world. Among them, pancreatic ductal adenocarcinoma (PDAC) is the most common, accounting for more than 90%. At present, surgical resection is the first choice for effective treatment. Adjuvant chemotherapy can improve long-term prognosis. However, most patients are already in the middle and advanced stages of diagnosis and lose the opportunity for surgery. Moreover, the effectiveness of existing chemotherapy options is not good, and the overall prognosis of patients is very poor.
One of the reasons for the predicament in pancreatic cancer treatment is the presence of abundant hydrated tissue in the extracellular matrix (ECM) of tumor cells. Hyaluronic acid (HA), the main component, hinders the delivery of chemotherapy drugs to tumor cells. In addition, many drugs with anti-cancer potential, such as the natural flavonoid quercetin (Que), can inhibit tumor cell growth but are limited in cancer treatment due to poor water solubility and low bioavailability.
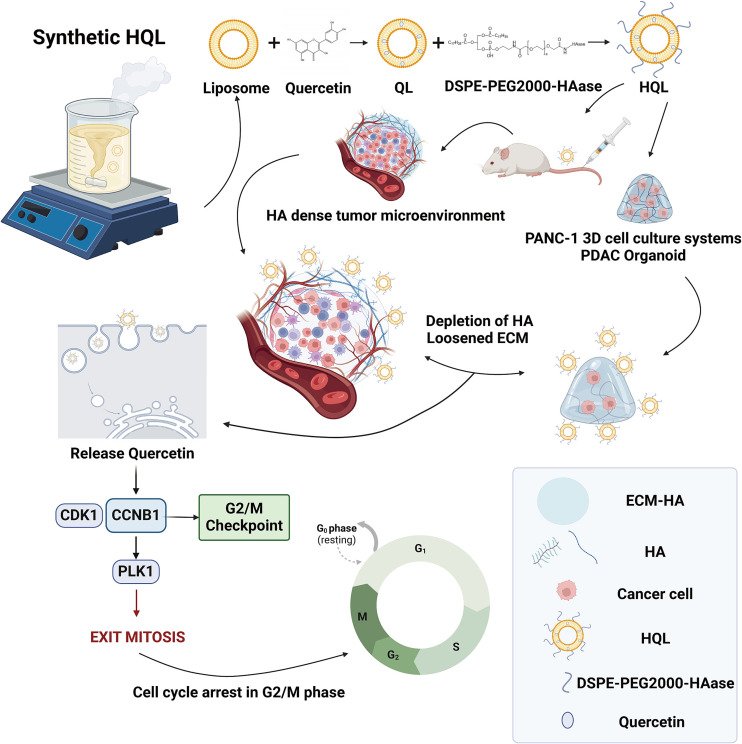
Recently, a study published in J Control Release entitled “Quercetin liposomes conjugated with hyaluronidase: An efficient drug delivery system to block pancreatic cancer” has brought new hope for pancreatic cancer treatment. The research team developed an efficient drug delivery system: quercetin was encapsulated into liposomes, and hyaluronidase (HAase) was conjugated to the surface of the liposomes. This novel liposome was named HQL.
Fig.1 The development process of HQL and its inhibitory activity on pancreatic cancer in vitro and in vivo.1
The researchers first detected HA content in pancreatic cancer tissues and various cell lines, finding that HA levels in pancreatic cancer tissues and cancer cell lines (such as PANC-1 and Miapaca-2) were significantly higher than those in normal tissues and other cancer cell lines. This result indicates that high HA content is an important characteristic of pancreatic cancer, providing a basis for subsequent HA-targeted treatment strategies.
After successfully preparing HQL, the researchers comprehensively characterized it. HQL is yellow, exhibits the Tyndall effect, has a particle size of 131.2±0.7 nm, a zeta potential of -13.29±0.25 mV, and an encapsulation efficiency of 96.6±2.98%. In vitro experiments showed that HQL could stably exist in 10% fetal bovine serum and plasma for 15 days. Its release rate was approximately 30% in an acidic environment (pH = 5.5) and about 20% in a neutral environment (pH = 7.4) within 4 hours, with sustained release for over 50 hours. In a simulated blood environment (pH = 7.4, 37°C), the release rate could reach nearly 100% after 10 hours, followed by continuous release. Meanwhile, the in vitro hemolysis rate of HQL was lower than 2%, confirming its safety in blood. Additionally, HAase in HQL remained highly active, laying the foundation for degrading HA in the tumor microenvironment.
In vitro cell experiments demonstrated that HQL had a significant proliferation-inhibiting effect on pancreatic cancer cells (PANC-1 and Miapaca-2), with an IC50 value lower than that of quercetin and other controls. It had no obvious inhibitory effect on the proliferation of normal pancreatic ductal epithelial cells (HPNE), showing good tumor cell specificity. HQL had stronger penetration ability in HA-rich agarose gels. In 2D and 3D cell culture models, it was more effectively taken up by pancreatic cancer cells, promoted cancer cell apoptosis, and induced cell cycle arrest at the G2/M phase. In experiments with 3D-cultured PANC-1 cells and PDAC organoids, HQL also showed superior ability to inhibit cell proliferation and promote apoptosis compared to other control groups.
In in vivo experiments, the researchers established a pancreatic cancer mouse model. Results showed that the tumor volume and weight in the HQL treatment group were significantly lower than those in the control group and other treatment groups, reducing tumor volume by 73% and decreasing HA levels in tumor tissues by 95%. Fluorescent labeling experiments revealed that HQL mainly accumulated in tumor tissues, with less distribution in organs such as the liver. The drug concentration of HQL in the blood could still be maintained at an effective level (50 μg/mL) within 48 hours, while the concentration of orally administered quercetin dropped to below 0.2 μg/mL after 48 hours. Furthermore, the in vivo enzymatic activity of HQL peaked at 60 U/mL at 2 hours, then gradually decreased and remained at approximately 20 U/mL within 48 hours. Safety evaluation indicated that HQL had no obvious impact on mouse body weight or the morphology and function of major organs (heart, liver, spleen, lung, kidney), showing no potential toxic side effects.
Mechanistic studies found that HQL may exert its effects of inhibiting tumor cell growth and promoting apoptosis by downregulating the expression of cell cycle-related proteins (CCNB1, CDK1, and PLK1) as well as apoptosis-related factors PI3K/AKT and Bcl-2.
In summary, the HQL liposomal drug delivery system developed in this study can effectively degrade HA in the pancreatic cancer tumor microenvironment, enhance drug penetration and delivery efficiency, significantly inhibit pancreatic cancer growth, and has good safety. It provides a new and promising drug delivery method for the adjuvant treatment of pancreatic cancer, which is expected to bring new hope to pancreatic cancer patients in the future and promote further development in the field of pancreatic cancer treatment.
With extensive experience in the field of bioconjugation, Creative Biolabs offers a comprehensive suite of services utilizing a variety of advanced conjugation strategies. Our expertise allows us to create stable and effective bioconjugates tailored to your specific research needs. We provide a diverse portfolio of technologies to ensure the optimal approach for your project.
- Lysine based Conjugation: A robust, conventional method targeting abundant lysine residues for attaching various molecules to proteins.
- Cysteine based Conjugation: Enables site-specific conjugation through free sulfhydryl groups for more homogeneous and precisely controlled bioconjugates like ADCs.
- Tyrosine-based Conjugation: An innovative, site-specific technique targeting tyrosine residues, minimizing disruption of the protein’s native function.
- EnCys-mAb based Conjugation: A proprietary antibody engineering approach that introduces cysteines for creating highly uniform immunoconjugates with a defined drug-to-antibody ratio (DAR).
- Enzyme-mediated Modification & Conjugation: Utilizes enzymes for highly specific conjugation under mild conditions, preserving the biomolecule’s integrity.
- Intein-fusion based Conjugation: A protein splicing technique for precise, post-translational insertion of molecules at specific sites within a protein.
- Meditope-based Conjugation: A novel, non-covalent method using a peptide to bind a payload to a specific antibody region without chemical modification.
- Carbohydrate-based Conjugation: Targets glycan moieties on glycoproteins for site-specific conjugation, preserving the protein’s active sites and antigen-binding affinity.
Reference
- Sun, Ge, et al. “Quercetin liposomes conjugated with hyaluronidase: an efficient drug delivery system to block pancreatic cancer.” Journal of Controlled Release 382 (2025): 113642. https://doi.org/10.1016/j.jconrel.2025.113642