PROTACs is a novel technique in protein drug research field. Unlike traditional protein inhibitors, PROTACs are potential to target undruggable protein. Therefore, it has become a potential cancer therapy strategy. PROTACs appears like a dumbbell consisting of a ligand (associated with POI), another ligand (binding to an ubiquitin ligase) and a linker (linked to two ligands). PROTAC recruits E3 ligase to POI (protein of interest) via ubiquitin proteasome system (UPS), labelling POI as defective or damaged protein via ubiquitin and caused POI degradation.
As the world’s first PROTACs-based molecular protein degradation agent ARV-110 revealed positive clinical data during I phase trials, this field rising in popularity. The treatment strategy of protacs to degrade various cancer targets has attracted the attention of a number of pharmaceutical and biotechnology companies.
Recently, a review focusing on new anti-cancer treatments based on PROTACs was reported in Nature’s Oncogene magazine. This article focuses on the potential of protacs as an anticancer therapy, chemical and bioinformatics methods designed by PROTAC, and safety issues, especially the development of tumor-specific / selective PROTAC.
Three types of PROTACs
The authors divided the mechanisms of PROTACs-mediated protein degradation into three types, as shown in the figure. Figure a is a schematic diagram of the general mechanism by which PROTAC induces the degradation of proteins of interest. Consistent with the previous description, PROTAC molecules recruit E3 ubiquitin ligase to the protein of interest, so that the protein of interest is labeled by a large number of ubiquitin molecules. After that, the polyubiquitinated protein of interest is recognized by proteasome and degraded. Then, the PROTAC molecules are recycled to induce the next round of degradation.
Figure b illustrates the mechanism of homo-PROTACs-mediated autodegradation of E3 ligase. An homo-PROTAC recruits an E3 ligase molecule (such as CRBN or VHL) to another E3 ligase molecule, followed by bidirectional polyubiquitin of the E3 ligase molecule, and then both molecules are degraded by proteasomes.
Figure c illustrates the mechanism of photocontrolled Photo-PROTACs-mediated degradation of proteins of interest. In photo-PROTAC, a photoremovable group is attached to an interest ligand or E3 ligase ligand or a “connector” (linker). After external light irradiation, the photomovable group was separated from photo-PROTAC and converted into active PROTAC, for protein of interest degradation.
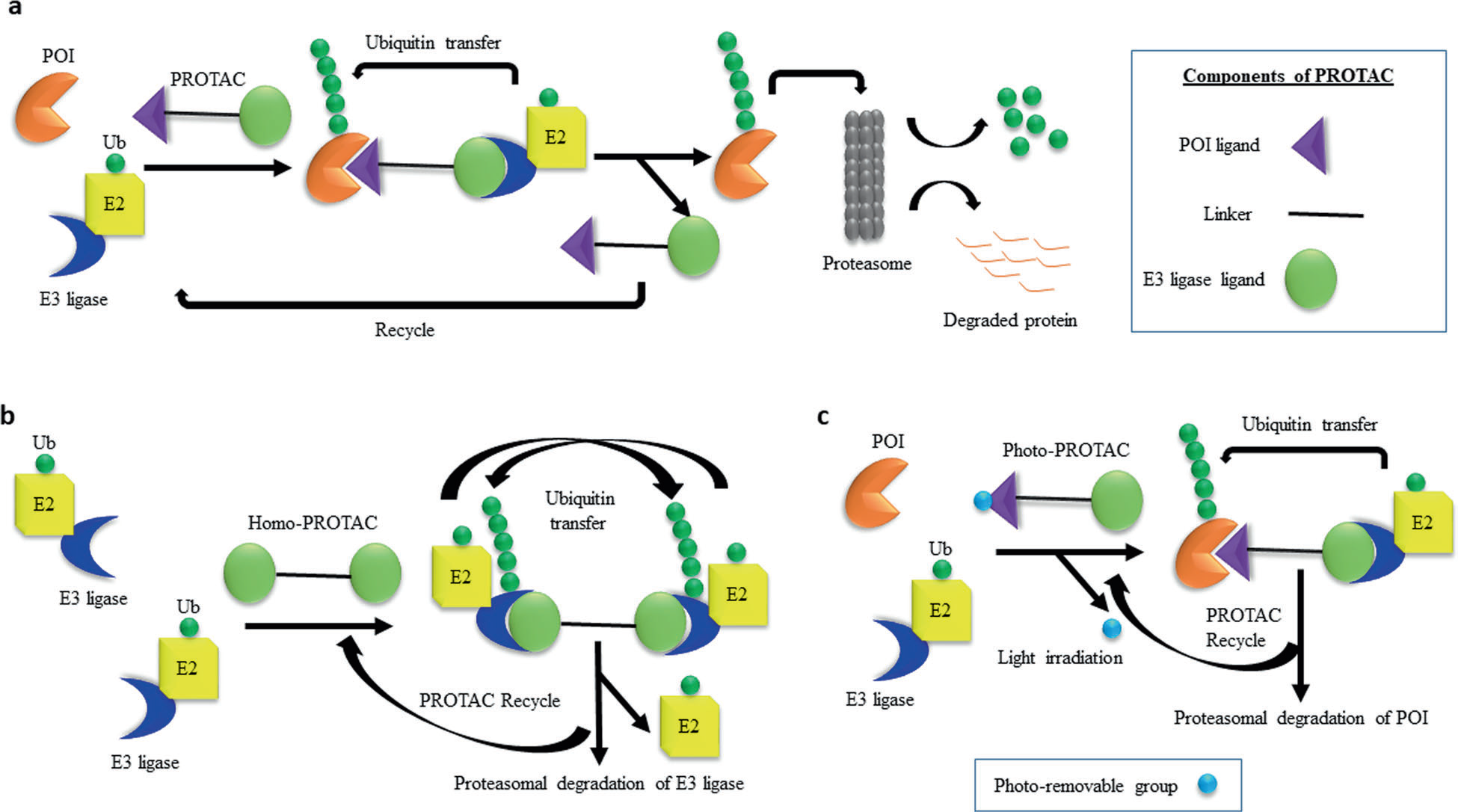 Fig.1 Mechanisms of PROTAC-mediated protein degradation. (Sajid Khan, 2020)
Fig.1 Mechanisms of PROTAC-mediated protein degradation. (Sajid Khan, 2020)
Commonly used E3 ligases and anticancer targets
Scientists have found more than 600 E3 ligases in the human genome, of which only a few have been used in PROTAC design. The development of anticancer PROTAC mainly uses the ligands of CRBN, VHL, MDM2, IAPs, DCAF15, DCAF16, RNF4 and RNF114 E3 ligase. Scientists have found more than 600 E3 ligases in the human genome, of which only a few have been used in PROTAC design. The development of anticancer PROTAC mainly uses the ligands of CRBN, VHL, MDM2, IAPs, DCAF15, DCAF16, RNF4 and RNF114 E3 ligase. Table 1 below summarizes the key anticancer targets for successful targeting of small molecule PROTACs developed with these E3 ligase ligands.
Advantages and disadvantages
Compared with the traditional small molecule inhibitor (SMIs), PROTACs has several advantages related to their unique mechanism of action. Its main advantages include the type of catalysis, high selectivity, the potential to target undruggable proteins and the ability to overcome drug resistance of small molecular inhibitors by targeting mutant proteins. In addition, PROTACs may achieve tumor-specific/selective degradation of the target protein by using ligands of tissue-specific and/or tumor-selective E3 ligase. Because of these properties, PROTACs avoids many of the side effects and limitations of small molecular inhibitors. However, before promoting clinical transformation, there are still some safety issues related to PROTACs (including on-target and off-target toxicity) need to be considered.
PROTACs has been developed for different targets against solid tumors and malignant blood cancers, and shows high efficacy against some tumor cells in a target-dependent manner. For example, PROTACs targeting BRD4, BTK, BCRABL and CDK-6 has shown the potential to treat leukemia, while ROTACs targeting AR, ER, FAK and P38 is being developed to treat a variety of different solid tumors. PROTACs targeting BCL-XL and ALK also showed broad-spectrum anti-tumor activity and could effectively kill leukemia and solid tumor cells both in vitro and in xenotransplantation models. However, unlike small molecule inhibitors, PROTACs has a larger molecular weight, thus, tissue permeability and cell permeability remain major challenges.
With the disclosure of some key clinical data, the development of new anticancer therapy based on PROTACs technology has entered a new stage. PROTACs and similar compounds may represent a new class of drugs different from chemotherapy, small molecular inhibitors, antibodies and cell therapy.
