Among the drugs developed in Arvinas, ARV-110 is the first oral PRAOTC small molecule drug to enter clinical trials in the field of proteolytic targeted chimerism, selectively targeting the degradation of androgen receptor (AR). In May 2019, ARV-110 received FDA fast-track approval for the treatment of metastatic trend-resistant prostate cancer (mCRPC).
Proteolysis targeting chimera (PROTAC) is a drug development technology that has attracted much attention at present. One end of these bifunctional small molecules is the ligand that targets the target protein, and the other end is the ligand that binds E3 ubiquitin ligase, which is then linked by a certain length of linker. E3 ubiquitin ligase can mark the target protein as defective or damaged protein by attaching a small protein called ubiquitin. After that, the cell’s protein shredder, proteasome, dispose of the labeled target protein.
The main advantages of PROTAC are as follows: targeted degradation of “unavailable drug targets” such as KRAS, STAT3, etc.; overcoming tumor drug resistance; prolonging the action time. PROTAC can not only affect the enzyme activity function of the protein but also regulate the non-enzyme activity function.
The history of this technology is only 20 years, but great progress has been made in the research and development of new drugs utilizing PROTAC. This time, as the first new targeted protein degrader in clinical trials in the world, the phase I results of ARV-110 have attracted a lot of attention because these vital data may really change people’s perception of whether PROTACs technology can be used as a medicine.
Metastatic castration-resistant prostate cancer (mCRPC) is the second-highest incidence of malignant tumor in men in Europe and the United States, and it has always been a difficult point in the treatment of prostate cancer and the focus of clinical research. At present, the first-line therapeutic drugs mainly targeting the androgen receptor (AR) are abiraterone and enzalutamide.
However, for patients with AR gene enhancer amplification or AR point mutation, the effect of first-line treatment is poor. 15-25% of the patients do not respond to second-generation hormone therapy such as abiraterone and enzalutamide, and most patients who are reactive will eventually develop severe drug resistance, resulting in a poor prognosis.
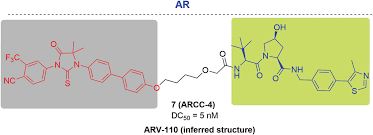
To overcome these challenges and improve current treatment options, ARV-110 was designed as a nuclear receptors ligand using ubiquitin-protease systems to degrade nuclear receptors AR proteins that play a key role in the development of prostate cancer, which is different from traditional inhibitors and does not need to “occupy” receptors to inhibit its function. In addition, the PROTAC drug can also work repeatedly to degrade newly transcribed target proteins, so it can overcome the increased expression of target proteins and mutations in target proteins. The specific molecular structure of ARV-110 has not been announced yet.
In preclinical studies, ARV-110 has shown promising activity as a targeted degradant of AR. In the model sensitive to enzalutamide, ARV-110 showed a decrease in prostate-specific antigen (PSA) similar to enzalutamide, and at a lower dose. In the Enzalutamide resistance model, ARV-110 could significantly inhibit tumor growth.
Clinical trials
In May 2019, FDA approved the phase I clinical trial to evaluate the safety, tolerance, pharmacokinetics, and pharmacodynamics of ARV-110 in patients with metastatic castration-resistant prostate cancer who had previously received at least two systemic therapies. In October, Arvinas released preliminary data from the ARV-110 Phase I trial (from 10 patients) at the second targeted protein degradants Summit. The results showed that the three dose groups (35 mg, 70 mg, and 140 mg) were well tolerated, and there was no dose limit toxicity, and no grade 2, 3, or 4 related adverse events were observed.
On May 13, 2020, the latest safety-related data and early efficacy data of Phase I Clinical trial (NCT03888612) were released in the summary of the American Annual meeting of Clinical Oncology (ASCO). In order to determine the maximum tolerable dose (MTD) and phase 2 recommended dose (RP2D) of ARV-110, mCRPC patients who had previously received at least two treatments (including enzalutamide and/or abiraterone) were given ARV-110 once a day.
As of January 2020, 2 of the 18 therapists had adverse reactions. In both patients, rosuvastatin and ARV-110 should be banned in view of the significant increase in plasma concentrations of rosuvastatin and AST/ALT at the same time. In addition, there were no other reports of level 3/4 adverse events (AE). Fifteen patients were assessed for PSA response, and 8 of them received an initial dose of 140mg. Two patients in the 140mg dose group were confirmed to have decreased PSA by more than 50%. These two groups of patients had previously received chemotherapy, bicalutamide and radium 223, as well as other treatments.
The latest clinical trial data show that ARV-110 has acceptable safety. The maximum tolerated dose (MTD) has not yet been established and the determination of phase 2 recommended dose (RP2D) is still in the trial.
