In recent years, targeted protein degradation chimera (PROTAC) as a novel way to induce protein degradation has become a new drug discovery strategy. Compared with traditional protein inhibitor drugs, it has unique technical advantages, and of course it also faces some new challenges.
1. Limitations of traditional drug discovery strategies
Traditional drug design often focuses on two principles of optimizing drug binding affinity and exposure, but it often limits the discovery of more efficient drugs, because it is not always easy to identify a highly effective and selective drug to regulate a biological target. In the face of the discovery of more and more drug intervention targets that lack high-affinity ligand binding sites, the traditional small molecule pharmaceutical technology seems helpless.
More and more methods have been used to circumvent these limitations. Although monoclonal antibodies and other protein inhibitors have the advantages of high affinity and selectivity, these inhibitors can only be used in extracellular or cell surface targets. However, most genomic proteins and contemporary drug targets play a role in cells.
2. Introduction of PROTAC technique
PROTAC technology was discovered by Israeli scientists Aaron Ciechanover, Avram Hershko and American scientist Irwin Rose while studying the process of ubiquitin-regulated protein degradation. They won the 2004 Nobel Prize in chemistry for this research.
The pharmacological mechanism of targeting protein degradation chimera is different from the traditional small molecule inhibitors. PROTAC adopts the pharmacological action mode of event-driven rather than the traditional small molecule occupancy-driven mode. The PROTAC molecule consists of two ligands and one linker, one ligand targets the interested protein, and the other ligand recruits ubiquitin E3 ligase, which is connected by a suitable linker. By recruiting ubiquitin E3 ligase to the surface of the target protein, PROTAC triggers the polyubiquitin process and induces the degradation of the target protein, so as to achieve the effect of disease treatment.
The mechanism of PROTAC is simple and clear, and the degradation of targeted specific proteins can be achieved through the body’s own ubiquitin-proteasome system. In theory, small molecular drugs could achieve efficient and specific protein degradation by just briefly binding to carcinogenic proteins and labeling carcinogenic proteins as “ubiquitinated”. These small molecular drugs do not need a high concentration as they can be recycled. After degradation, the target protein needs to be re-synthesized to restore function, which greatly delays the emergence of drug resistance and overcomes the shortcomings of traditional methods such as CRISPR, RNAi and small molecular inhibitors. Therefore, PROTAC technology has been paid more and more attention in the research and development of new drugs.
3. The Development of PROTAC
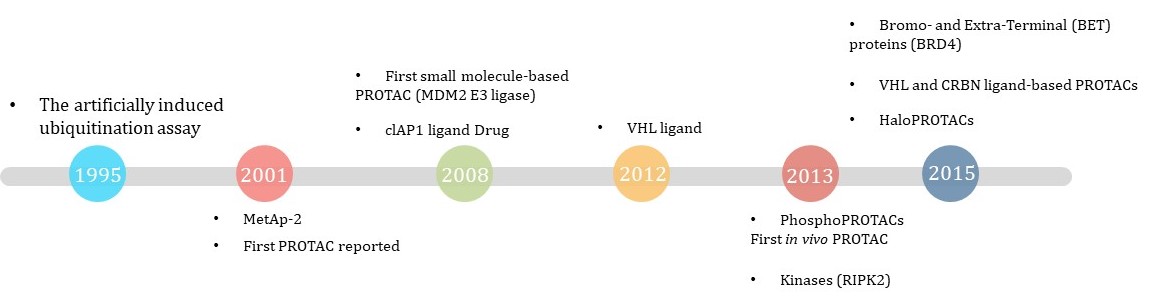
In 1995, the earliest reports of artificially induced protein degradation was the design of a series of modified E2-binding enzymes (such as TaUBC4) fused with Ig-binding motifs, which showed for the first time that artificially induced ubiquitination could be recognized by degraded proteasomes. In 2001, Deshaies and Crews laboratories used the E3 ubiquitin enzyme containing SCF β-TRCP to induce the degradation of MetAp-2 and used the word protac for the first time.
This early groundbreaking paper selected an ovalbumin derivative that binds to Met-AP2 through covalent interactions. This dependence on covalent bonds prevents one of the unique advantages of PROTAC, that is, its catalysis, which potentially limits the observed degradation efficiency. In the next few years, PROTAC developed to be able to degrade targets such as estrogen receptor (ER) and androgen receptor (AR). Because the early PROTAC molecules are mostly small peptides, its cell permeability is poor, and its cell activity is also low, which makes it cannot be used in drug development.
Until 2008, the Crews team reported small molecule PROTAC-nutlin for the first time. Nutlin induces the degradation of androgen receptor (AR) by binding to E3 ligase Mdm2, but its cellular activity is still not ideal. AR degradation is only induced at the concentration of 10 μM. At the same time, although the reported data show that the degradation is proteasome-dependent, it is considered that many AR ligands can spontaneously destroy the stability of their homologous receptors and cause self-ubiquitin and degradation. Therefore, its application in drug discovery is still limited.
From 2010 to 2012, the discovery of more ligands of ubiquitin E3 ligase greatly promoted the research progress of PROTAC. In 2012, the high affinity peptide-like ligands of VHL were discovered. Subsequently, the, Alessio Ciulli team reported the structure-activity relationship of VHL peptide-like ligands.
In 2013,phosphoPROTACs,the first in vivo PROTAC, was developed by Craig M. Crew’s group.
After 2015, small molecule PROTAC technology has gradually matured and become a real method for drug discovery, which has attracted the attention of academia, industry and investment institutions.
4. Advantages of PROTAC technology
- Catalytic degradation function
Protac plays a catalytic role in the degradation of target protein, so a good degradation efficiency can be achieved with low compound concentration.
- Selectivity
In addition to be a catalysis, PROTAC could selectively act on specific target proteins.
- A wide range of targets
Ubiquitin-proteasome system is the main pathway of intracellular protein degradation, which participates in more than 80% of intracellular protein degradation. E3 ubiquitin ligase is widely expressed in many kinds of cells. PROTAC molecule only needs to draw the target protein close to E3 ubiquitin ligase, and then degrade the target protein by proteolysome. Therefore, PROTAC technology can be widely used in different targets.
- Prolong the action time
The degradation of the target protein is time-dependent, and PROTAC can consume the intracellular target protein close to the basic level in a few minutes. New protein synthesis takes a long time which greatly prolong the effective time of PROTAC.
- Novel model of pharmacological action
In the past, many protein targets were considered to as non-druggable molecular due to the lack of specific catalytic active sites. But PROTAC technology provides a solution for these non-druggable molecules.
