RSV: The “Respiratory Killer” Lurking Among Us
When discussing respiratory viruses, people are often more familiar with influenza or COVID-19, but RSV (Respiratory Syncytial Virus) is actually a hidden “health threat,” particularly for two groups: infants and the elderly. Newborns have immature immune systems, narrow airways, and soft cartilage; once infected with RSV, they are prone to airway obstruction and severe lower respiratory tract infections. Conversely, as the elderly age, their immune function declines (“immunosenescence”), and combined with underlying conditions like chronic obstructive pulmonary disease (COPD) or cardiovascular disease, infections often result in more severe illness.
Additionally, immunocompromised individuals are also a high-risk group. Regarding the virus itself, RSV is a single-stranded negative-sense RNA virus with a genome of approximately 15.2kb, encoding 9 structural proteins and 2 non-structural proteins. Among these, the F protein and G protein are the “key weapons” for the virus to invade the human body—both are transmembrane glycoproteins responsible for mediating viral binding and fusion with host cells, making them core targets for vaccine development.
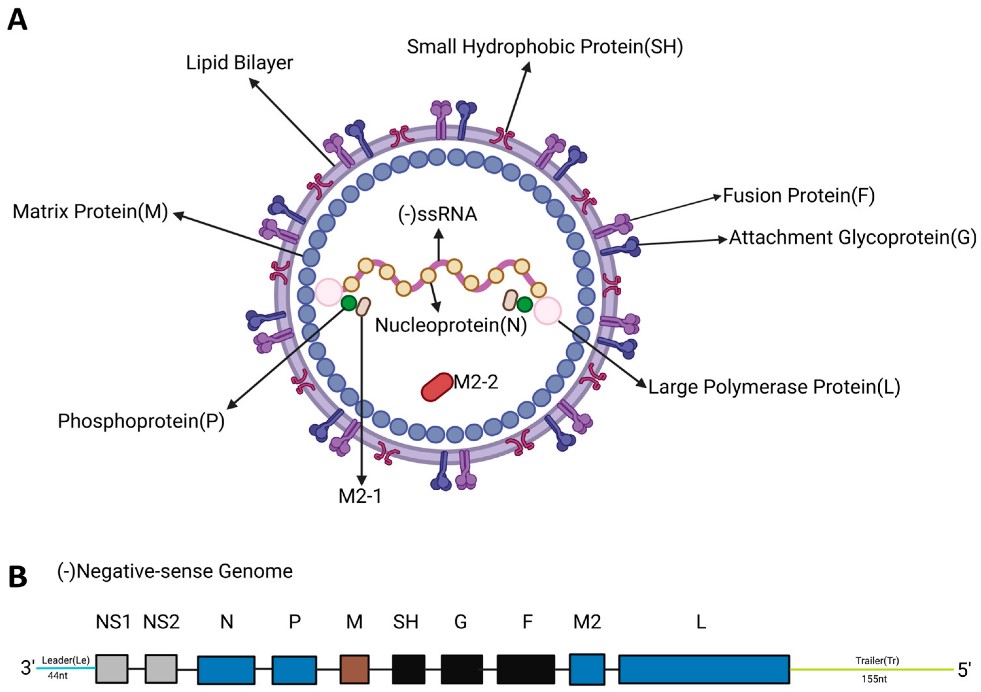
Fig.1 Overview of the RSV genome and viral architecture.1
The Core of Vaccine R&D: The “Structural Codes” of F and G Proteins
To develop effective RSV vaccines, one must first understand the “weaknesses” of the F and G proteins. Although both participate in viral invasion, their structures and characteristics differ significantly, determining different vaccine design strategies.
- F Protein: Stabilizing the “Prefusion Conformation” is Key
The F protein is one of the most conserved proteins in RSV; the extracellular domains of subtypes A and B differ by only 25 amino acids, meaning a vaccine targeting it could cover more strains. It has two key conformations: Prefusion (PreF) and Postfusion (PostF).
PreF is the state before the virus invades the cell, exposing multiple neutralizing epitopes on its surface, especially the Ø and V sites, which can induce potent neutralizing antibodies. In contrast, PostF is the stable state after fusion is complete, with significantly fewer neutralizing epitopes and greatly reduced immunogenicity. A major reason for the failure of early formalin-inactivated vaccines (FI-RSV) was the failure to preserve the PreF conformation of the F protein, resulting in vaccines that not only lacked protection but also potentially caused more severe disease. Therefore, a core technology of modern RSV vaccines involves using molecular engineering—such as introducing disulfide bonds, filling internal cavities, or fusing trimerization domains—to stabilize the F protein in the PreF conformation.
- G Protein: Targeting the “Conserved Core Domain” to Tackle Mutation
Unlike the highly conserved F protein, the G protein is the most rapidly mutating protein in RSV, with variations concentrated in the mucin domains at both ends. However, luckily, there is a Central Conserved Domain (CCD) in the middle of the G protein containing a CX3C motif, which binds to the CX3CR1 receptor on human cells and is key for viral attachment. This conserved domain has become a core target for G protein vaccines—even if the virus mutates, the structure of the CCD domain remains relatively stable, capable of inducing cross-strain immune protection.
Additionally, the G protein can interfere with human immunity by mimicking chemokines, so vaccines targeting it may simultaneously inhibit viral invasion and immune evasion.
Challenges and Future: What Hurdles Remain for RSV Vaccines?
Despite significant achievements in RSV vaccine R&D, many challenges remain.
First is the stability of the PreF conformation—even with engineering, some vaccines may still undergo conformational changes during storage or transport, affecting immune efficacy. Second is immunosenescence; as immune function declines in the elderly, designing vaccines suitable for them (e.g., optimizing adjuvants, adjusting doses) remains key. Furthermore, safety risks (like GBS, preterm birth risks) and cold chain dependence limit global accessibility, especially in low- and middle-income countries.
In the future, RSV vaccine R&D may focus on three directions: developing PreF+G protein combination vaccines to cover more neutralizing epitopes and improve protection breadth and durability; optimizing VLP technology combined with multi-antigen designs to enhance immunogenicity; and improving vaccine stability and cold chain requirements to facilitate easier distribution. At the same time, further clarification of immune protection mechanisms is needed to avoid relying solely on in vitro neutralizing antibody data to judge vaccine efficacy.
Conclusion
From the failure of early inactivated vaccines to the approval of multiple vaccines today, RSV vaccine R&D has traversed a path of exploration spanning over half a century. Technologies like F protein PreF conformation stabilization, G protein conserved domain targeting, and novel vectors like VLPs have brought us closer to comprehensive RSV prevention and control.
For the general public, understanding the target populations and technical characteristics of RSV vaccines can help us better protect our families—high-risk groups like the elderly and pregnant women can receive marketed vaccines based on medical advice, while innovative breakthroughs in pipeline vaccines offer the possibility of safer, more convenient protection in the future.
Accelerating RSV Research with Creative Biolabs
Creative Biolabs leverages extensive expertise in vaccine development to provide a comprehensive suite of services and products designed to advance respiratory syncytial virus (RSV) research. We support researchers at every stage, from antigen discovery to formulation development, ensuring robust and scalable solutions for this critical respiratory pathogen.
- Human Respiratory Syncytial Virus Vaccines: We offer specialized development services tailored to human RSV, addressing the unique challenges of immunogenicity and safety in vaccine design.
- Bovine Respiratory Syncytial Virus Vaccines: Our capabilities extend to veterinary medicine, providing dedicated vaccine solutions for Bovine RSV to support animal health and agricultural productivity.
Research-Grade Products: To facilitate your experimental needs, we provide high-quality reagents, including Inactivated RSV Antigen, essential for assay development and immunological studies.
Reference
- Yu, Dongrunhan, et al. “RSV Vaccines: Targeting Prefusion F and G Proteins from Structural Design to Clinical Application.” Vaccines13.11 (2025): 1133. CC BY4.0. https://doi.org/10.3390/vaccines13111133
