The world watched in real-time as a new class of medicine, once confined to research labs, emerged to combat a global pandemic. We’re talking about messenger RNA (mRNA) vaccines, and their success over the last few years has been nothing short of a revolution in medicine. Beyond their rapid development and deployment for viral outbreaks, these technologies are now unlocking a future filled with potential—from new ways to fight cancer to treatments for infectious diseases that have long plagued humanity.
But what exactly are mRNA vaccines, and why are they so powerful?
At its core, an mRNA vaccine doesn’t contain a weakened virus or a piece of a protein. Instead, it contains a tiny blueprint—a set of instructions—that tells our own cells how to create a specific protein. For a viral vaccine, this protein is usually a harmless piece of the virus, like the “spike protein.” Once our cells produce this protein, our immune system recognizes it as foreign, learns to create a defense against it, and then remembers that defense for the future. The mRNA itself is quickly degraded by the body, leaving behind only the immunity we’ve built. It’s a remarkably clean and elegant approach.
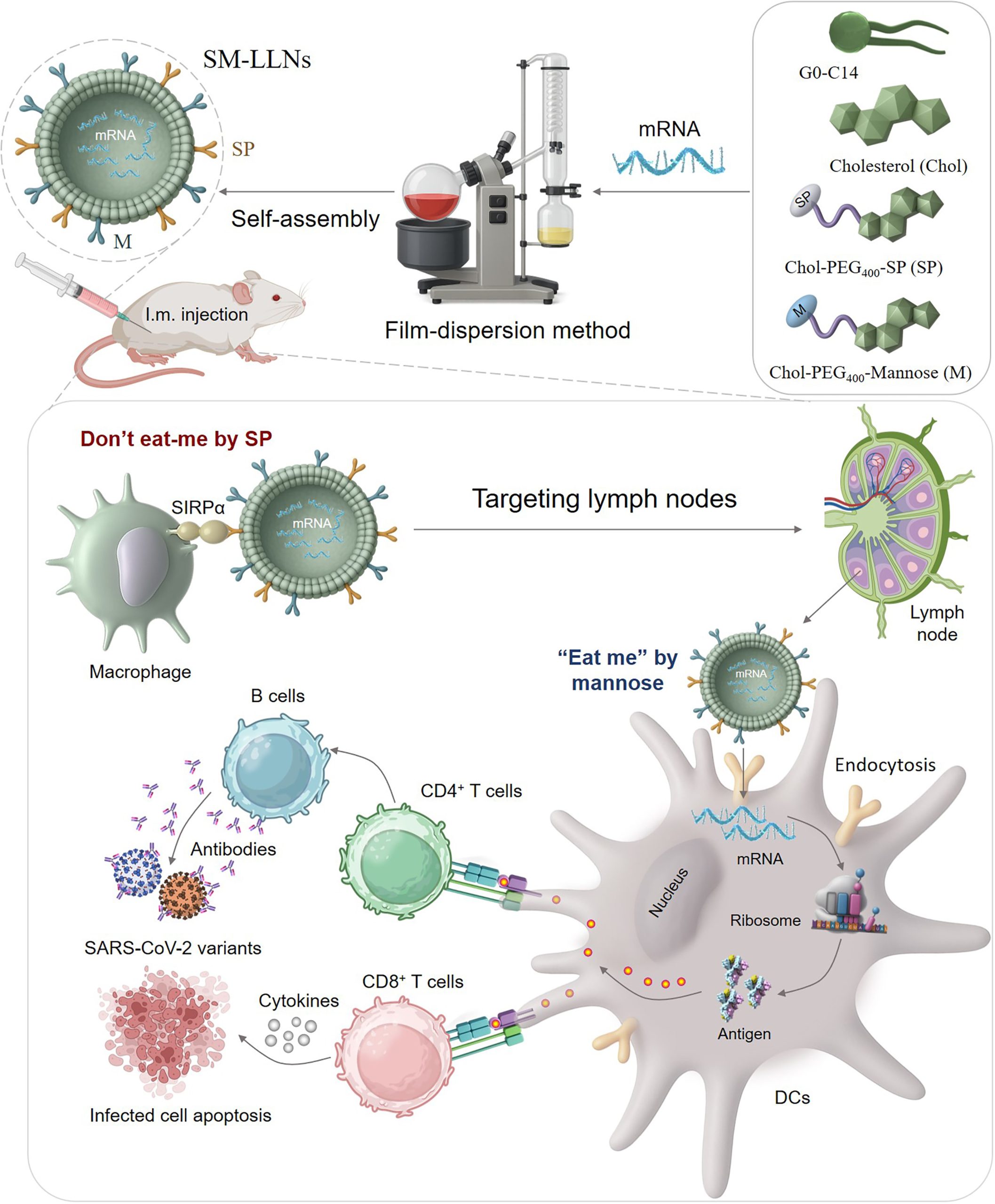
Fig.1 Diagram Illustrates the Process of A Precision-Engineered mRNA Nanovaccine.1
The Great Challenge: Getting the Message Across
For all their promise, mRNA vaccines have a significant hurdle: the mRNA molecule is fragile and can’t just be injected on its own. It needs a sophisticated delivery system to protect it and ensure it gets to the right cells. Think of it like a secret message that needs to be delivered to a specific address, but the mail carrier can’t read it, and it will dissolve in the rain.
Historically, this has been a major challenge. The mRNA has to be shielded from enzymes in the body that would rapidly destroy it, and it has to be guided to the precise type of cell that can best present the viral protein to the immune system.
One of the most promising solutions has been the use of lipid-like nanoparticles (LLNs). These tiny, fatty spheres act as a protective courier, encasing the fragile mRNA and navigating it through the body’s complex biological landscape. The success of these delivery systems has been a game-changer, but researchers are constantly working to make them even better. The goal is to maximize their delivery to key immune cells—specifically dendritic cells (DCs)—while minimizing their uptake by other cells that might clear the vaccine before it can do its job.
A Smarter Approach: The ‘Eat-Me’ and ‘Don’t-Eat-Me’ Strategy
Recent research has unveiled a clever, dual-ligand strategy to optimize this delivery process. This approach essentially gives the LLNs a set of two signals: one that says “don’t eat me” and another that says “eat me.”
The “don’t-eat-me” signal is achieved by adding a specific peptide derived from the CD47 protein to the nanoparticle’s surface. This peptide, known as a self-peptide (SP), interacts with a receptor on the surface of macrophages—the body’s “clean-up crew.” By triggering this inhibitory signal, the nanoparticles are essentially camouflaged, preventing macrophages from non-specifically clearing them out of the bloodstream. This prolongs the vaccine’s circulation time, giving it more opportunities to reach the intended target cells.
Concurrently, a second “eat-me” signal is incorporated using a mannose ligand. Mannose is a sugar that is specifically recognized by mannose receptors, which are found in abundance on dendritic cells (DCs). This signal acts like a targeted homing beacon, actively directing the nanoparticles to DCs. This dual-signal approach—repelling off-target cells while attracting the right ones—is a powerful way to ensure the vaccine payload is delivered efficiently to the cells that matter most for initiating a strong immune response.
From Lab to Living System: The Proof is in the Response
The results of this dual-targeting strategy are significant. Studies have shown that nanoparticles with both of these modifications, referred to as SM-LLNs, are highly effective. When injected, these nanoparticles successfully deliver their mRNA payload to the lymph nodes, which are the central command centers of the immune system. This targeted delivery leads to a substantial increase in mRNA expression and protein production within the lymph nodes.
This isn’t just a technical achievement; it has real-world immunological consequences. The efficient delivery and expression of the mRNA cause the dendritic cells to mature, essentially putting them on high alert. These activated DCs then go on to present the newly created viral proteins to other immune cells, like T lymphocytes.
The result is a robust and long-lasting immune response. Not only does the body produce a high level of neutralizing antibodies—the B cell-mediated part of immunity—but it also generates a potent and biased T helper type 1 (Th1) cellular response. A Th1-biased response is crucial for effectively fighting and clearing viral infections. What’s more, these tailored nanoparticles have demonstrated a favorable safety profile with no significant signs of toxicity in studies.
Beyond Vaccines: A World of Possibilities
This leap forward in delivery technology has implications far beyond infectious disease vaccines. The ability to precisely target specific cell types with an mRNA payload opens up a vast new frontier in medicine.
Imagine applying this same principle to other diseases. Instead of a viral protein, the mRNA could contain instructions for a therapeutic protein, an enzyme that’s missing due to a genetic disorder, or an antigen that trains the immune system to attack cancer cells. The same dual-signal strategy could be used to direct these treatments to the right cells, whether they are a specific type of immune cell for immunotherapy or a diseased cell that needs repair.
The progress is breathtaking. From fighting new viral variants to offering hope for personalized cancer treatments and genetic therapies, the advancements in mRNA delivery systems are paving the way for a truly transformative era of medicine. It’s a testament to the power of targeted engineering and a reminder that the most revolutionary breakthroughs often lie in solving the most fundamental challenges. The mRNA revolution is just beginning, and with each new innovation in delivery technology, its potential grows exponentially.
Reference
Qin, Shugang, et al. “A precision-engineered dendritic cell-targeted mRNA nanovaccine for enhanced antiviral immunity.” Cell Biomaterials (2025). CC BY 4.0. https://doi.org/10.1016/j.celbio.2025.100180
