Introduction Fc Engineering Published Data
Equipped with the latest techniques and years of expertise in the antibody engineering field, Creative Biolabs provides advanced Fc Engineering in Bispecific Antibody (BsAb) service for optimizing BsAb development. We guarantee tailored modifications to enhance efficacy, stability, and immunogenicity, ensuring improved therapeutic potential. Partner with us to accelerate your BsAb research.
The Role of Fc
The Fc region of an antibody mediates effector functions like ADCC and CDC by binding to Fc receptors on immune cells. It also contributes to the antibody's pharmacokinetic properties, influencing its serum half-life through interactions with the neonatal Fc receptor (FcRn).
Fc Engineering Necessity for BsAb
Fc Engineering for Enhanced Fc-Effector Functions
Fc engineering aims to amplify or diminish BsAb effector functions by modifying Fc glycosylation or amino acid sequences. Enhancing ADCC involves increasing binding affinity to activating Fc receptors, such as FcγRIIIa, often achieved by removing fucose from N-linked glycans or introducing specific mutations. Conversely, reducing binding to inhibitory Fc receptors strengthens the overall response. For example, mutations that increase FcγRIIIa affinity can significantly boost tumor cell killing. Glycoengineering, like producing afucosylated antibodies, is a common strategy. Tailoring these modifications allows for precise control over immune activation, optimizing BsAb potency for targeted therapies in oncology and other diseases. The goal is to maximize therapeutic impact while minimizing off-target effects.
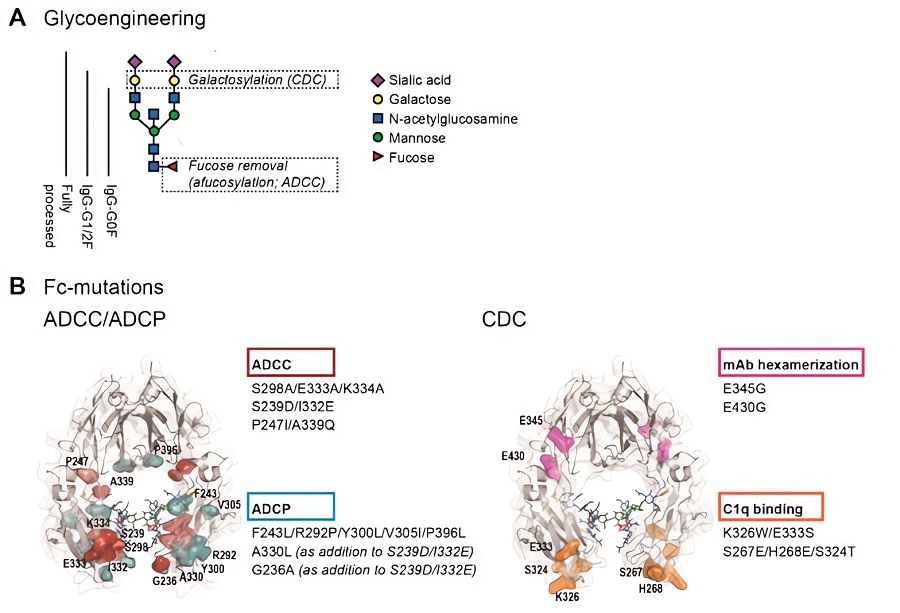 Fig.1 Fc engineering methods to enhance Fc-effector functions.1
Fig.1 Fc engineering methods to enhance Fc-effector functions.1
Fc Engineering for Pharmacokinetics and Half-Life Extension Optimization
The Fc region's interaction with FcRn is fundamental for regulating BsAb serum half-life. Fc engineering focuses on optimizing this interaction to extend circulation. Mutations that increase FcRn binding at acidic pH, prevalent in endosomes, while maintaining or reducing binding at neutral pH, in the bloodstream, are favored. The YTE (M252Y/S254T/T256E) mutations are widely used, significantly enhancing FcRn binding and prolonging half-life. Extended half-life reduces dosing frequency, improves patient compliance, and potentially lowers treatment costs. This strategy is critical for ensuring sustained therapeutic concentrations and maximizing BsAb efficacy in chronic diseases.
Fc Engineering for Bispecific Heterodimeric IgG-Based Antibodies Formation
Generating heterodimeric BsAbs requires precise control over heavy-chain pairing. Fc engineering, notably "knobs-into-holes" technology, facilitates this. By introducing complementary mutations into the CH3 domains, the formation of heterodimers is promoted over that of homodimers. This ensures that the BsAb has the desired dual-targeting capabilities. The "knob" mutation in one CH3 domain preferentially interacts with the "hole" mutation in the other, promoting correct pairing. This approach is essential for producing functional BsAbs with the correct binding specificities, critical for therapeutic applications. Further optimization aims to increase stability and reduce potential immunogenicity.
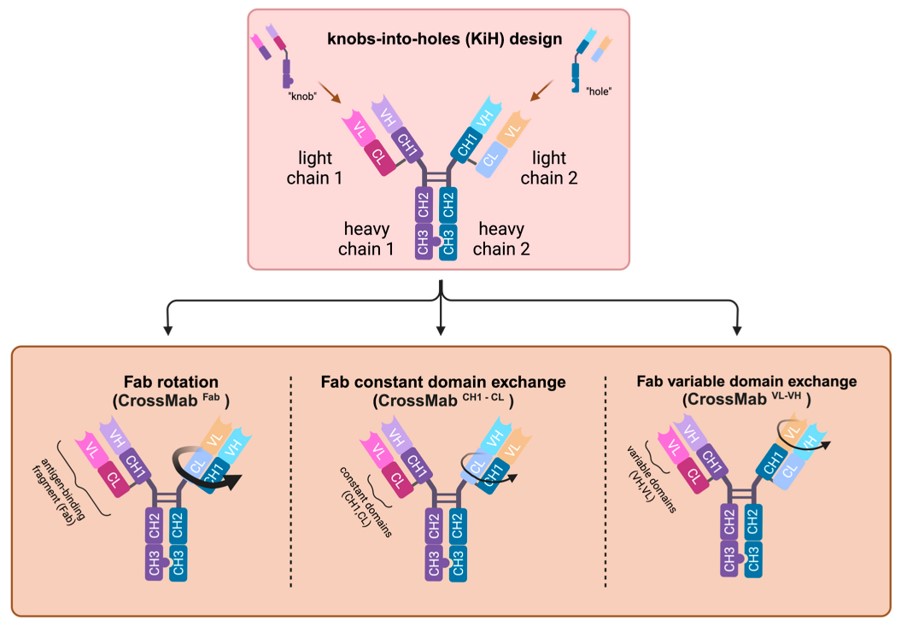 Fig.2 "Knob-into-holes" and CrossMab design of heterodimeric BsAbs.2,4
Fig.2 "Knob-into-holes" and CrossMab design of heterodimeric BsAbs.2,4
Published Data
1. Engineered IgG Fc Region with Dual Mutations Results in Half-Life Extension Optimization and Effector Function Enhancement
This study used directed evolution to identify Fc variants that enhance pH-dependent binding to human FcRn, improving the serum half-life of an Fc-fusion protein and a model IgG antibody. The engineered Fc variants resulted in markedly longer serum extensions of human FcRn transgenic mice compared to wild-type Fc. Additionally, in a cynomolgus monkey model, the Fc-bound antibody showed an improved pharmacokinetic profile. Two mutations in PFc29 also boosted CDC and ADCC activity, which are essential for cancer cell clearance. Furthermore, combining these mutations with effector function-silencing mutations allowed for controlled activation of these functions while maintaining optimal pH-dependent FcRn binding. These Fc variants offer a promising tool for improving the pharmacokinetics and therapeutic efficacy of antibodies and Fc-fusion proteins.
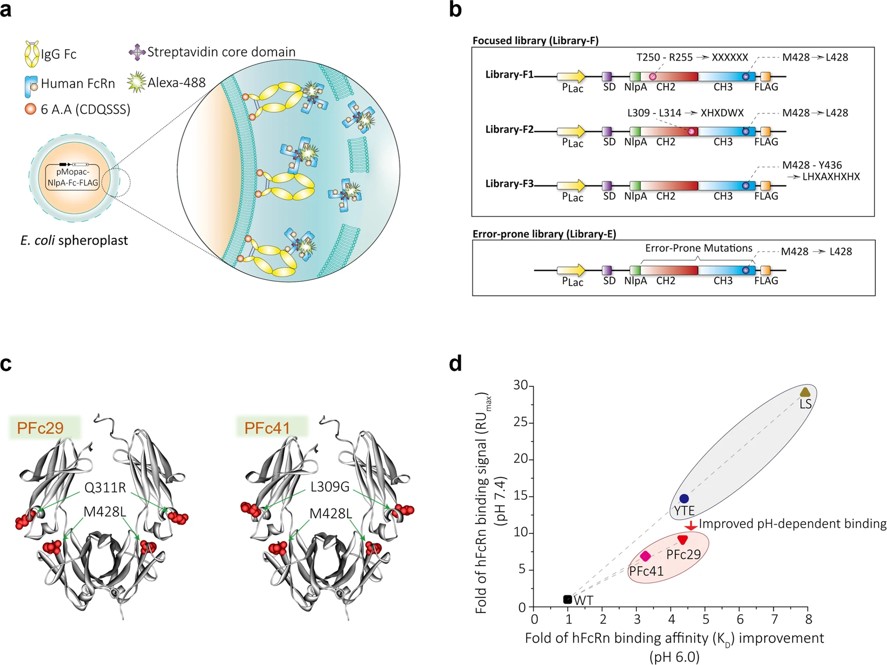 Fig.3 Isolation of Fc variants with improved FcRn binding.3,4
Fig.3 Isolation of Fc variants with improved FcRn binding.3,4
Creative Biolabs has multidisciplinary scientists and robust platforms dedicated to tailoring BsAb development to support your research needs. Through our diverse Fc engineering strategies, we are confident that we can provide you with a BsAb that meets your specific requirements.
References
-
Van der Horst, Hilma J., et al. "Fc-engineered antibodies with enhanced Fc-effector function for the treatment of B-cell malignancies." Cancers 12.10 (2020): 3041. Distributed under an Open Access license CC BY 4.0. The image was modified by extracting and using only part of the original image.
-
Abdeldaim, Dalia T., and Katharina Schindowski. "Fc-engineered therapeutic antibodies: recent advances and future directions." Pharmaceutics 15.10 (2023): 2402.
-
Ko, Sanghwan, et al. "An Fc variant with two mutations confers prolonged serum half-life and enhanced effector functions on IgG antibodies." Experimental & Molecular Medicine 54.11 (2022): 1850-1861.
-
Distributed under an Open Access license CC BY 4.0, without modification.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY


 Fig.1 Fc engineering methods to enhance Fc-effector functions.1
Fig.1 Fc engineering methods to enhance Fc-effector functions.1
 Fig.2 "Knob-into-holes" and CrossMab design of heterodimeric BsAbs.2,4
Fig.2 "Knob-into-holes" and CrossMab design of heterodimeric BsAbs.2,4
 Fig.3 Isolation of Fc variants with improved FcRn binding.3,4
Fig.3 Isolation of Fc variants with improved FcRn binding.3,4


