All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Lentiviral vectors (LVs) are currently one of the most commonly used vectors in clinical trials. In adoptive T cell cancer therapy, autologous T lymphocytes are genetically modified by LV to express T cell receptor (TCR) or chimeric antigen receptor (CAR) to expand or redirect their antigen spectrum to malignant cells. However, the industrial production of LV mainly depends on transient transfection. The use and development of a new LV packaging and production cell lines provide an easier scale-up, reproducibility, and cost-effective approach for LV scalable manufacturing.
At Creative Biolabs, we have developed a highly efficient LentiStable™ constitutive stable PCL platform by using a constitutive system based on replacing the VSV-G envelope to avoid cytotoxicity. Our advanced automation technology allows us to simplify production, minimize process risks and reduce costs. Our proprietary robotic system uses the most advanced automated technology to screen and isolate up to 3000 clones, thereby enabling high-titer lentiviral vectors can be confirmed in a short period to produce clones. These facilities make LentiStable™ a very promising and stable PCL for large-scale LV production.
Several steps are required to obtain a transfusible viral vector (Figure 1). The first is to cultivate and expand the packaging cell line (PCL) to obtain a large enough cell population. Traditionally, HEK293 is easy to transfect and adapts to different culture strategies, making it very suitable for LV production. The next step is to transfect the plasmid into the packaging cells, which will produce vector particles. Finally, before the product is collected, purification and filtration steps are critical.
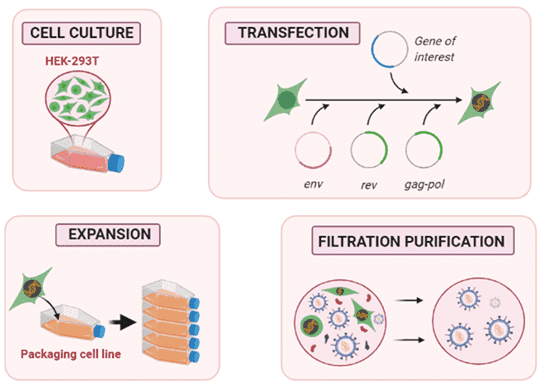 Fig.1 Proposed steps for lentiviral vector production in a packaging cell line.
Fig.1 Proposed steps for lentiviral vector production in a packaging cell line.
According to your specific needs, Creative Biolabs' expert team can design your producer cell line. Compared with traditional transient transfection methods, using our stable production cell line can achieve higher titers of lentiviral vectors. Since helper viruses, expensive transfection reagents or cGMP grade plasmids are not required, the final virus will be delivered in significantly reduced timelines.

All components produced by LV are stably integrated into one producer cell line

Scale up from shake flasks to large bioreactors without reducing productivity

Reliably produce LV in suspension bioreactors and serum-free media

Consistent LV quality from batch to batch-no transfection step
Stable packaging cells that can maintain constitutive and high-titer LV production are ideal systems for industrial large-scale continuous processes. In contrast to inducible cell lines, constitutive systems allow continuous expression of all vector components without cytotoxicity limitations. The ultimate advantage is that multiple carriers can be harvested, extended production periods, and an overall decrease in LV manufacturing costs, thereby providing more cost-effective LV Scalable production.
If you are interested in our services, please send an email to contact us, and our team will get back to you as soon as possible.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION