All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Lentiviral vectors (LV) represent a key tool for gene and cell therapy applications. However, the production of these vectors is plasmid supply-dependent and cannot be directly expanded. The production of sufficient quantities of viral products for clinical applications remains an obstacle and has prompted the development of suspension processes in this field that are conducive to mass production.
At Creative Biolabs, we use an inducible system to avoid cell death due to the continuous expression of cytotoxic proteins (Gag, Rev, and VSV-G), thereby developing a highly efficient LentiStable™ inducing stable PCL platform. The LV packaging cell line can stably integrate certain components of the lentiviral system into the cell genome, thereby achieving scalable and cost-effective manufacturing.
We have generated suspension and adherent-based stable packaging and producer cell lines using an inducible system. The packaging cell line stably integrates certain components of the lentiviral system into the cell genome (envelope (e.g. VSV-G), Gag/Pol and Rev), while the production cell line integrates all components (envelope (e.g. VSV-G), Gag/Pol, Rev, and Vector Genome). Since this process does not require helper viruses, expensive transfection reagents, or cGMP-grade plasmids, it can achieve stable high-quality virus delivery, cost-effective manufacturing, and minimize process risks.
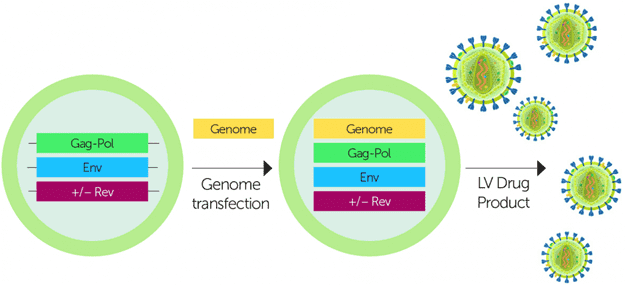 Fig.1 Schematic for lentiviral vector production in an inducible packaging cell line.
Fig.1 Schematic for lentiviral vector production in an inducible packaging cell line.

All components produced by LV are stably integrated into one producer cell line

Scale up from shake flasks to large bioreactors without reducing productivity

Reliably produce LV in suspension bioreactors and serum-free media

Consistent LV quality from batch to batch-no transfection step
If you are interested in our services, please send an email to contact us, and our team will get back to you as soon as possible.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION