Scientists from Creative Biolabs are willing to provide custom cell line affinity measurement using Surface Plasmon Resonance microscopy (SPR microscopy). It is a state of the art label-free technique, which combines the advantages of SPR with electron microscopy to study the ligand-receptor interaction of living cells in real-time monitor.
Technically, it is challenging to measure the binding kinetics of membrane proteins with living cells. Traditional methods such as fluorescence-based technology is a label based end-point assay that measures the changes of fluorescence signals before and after binding without the obtaining of binding kinetics. However, the label-free techniques, such as SPR, can measure protein-binding kinetics by immobilizing purified proteins onto a SPR chip. But for membrane proteins, an amphiphilic lipid bilayer has to be introduced to keep membrane proteins embedded into the liposome to preserve their activity. Meanwhile, atomic force microscopy can provide high spatial resolution for the study of membrane proteins, however, it is not suitable for the study of binding kinetics.
Nowadays, surface plasmon resonance microscopy (SPRM), a label-free imaging method, allows both optical and fluorescence imaging of the same sample without extracting membrane proteins from living cells. Briefly, cells are first cultured on a gold film, which is placed in an inverted microscope for simultaneous bright-field, fluorescence and SPRM imaging. Then, a p-polarized laser beam is casted onto a gold-coated glass coverslip through an oil-immersion objective to create SPR on the gold medium surface, which is imaged by a CCD camera. Additionally, a mercury lamp and appropriate optical filters are used to perform epifluorescence measurements. Furthermore, conventional bright-field images of the same sample can also be taken by illumination from the top of the sample.
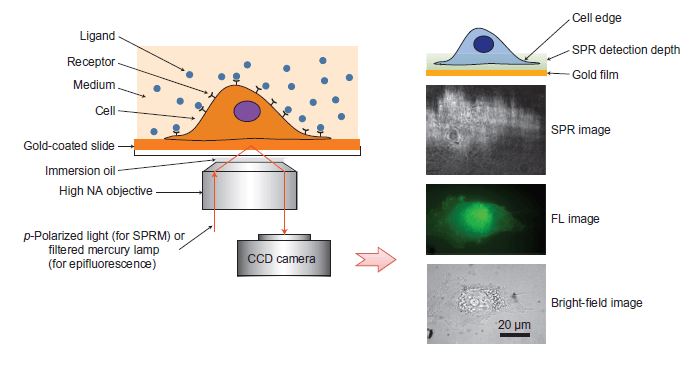 Figure 1. Surface plasmon resonance microscopy
Figure 1. Surface plasmon resonance microscopy
Like traditional SPR approaches, ligand-binding can result in the changes of mass concentration close to the surface. Therefore, the associate constant (Ka), disassociated constant (Kd) and binding affinity (KD) can be determined from the signal response unit (RU) changes.
SPR microscopy is capable of working out the measurement of binding kinetics between ligands and receptors in single living cells. The sensorgrams can be fitted pixel by pixel with a mathematic model to map the local association and disassociation constant. Thus, the real-time distribution images of ligands’ interaction with cells can be obtained in a non-invasive way to get the insight of dynamic characteristics of the molecules of interest.
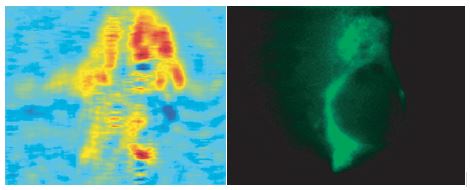 Figure 2. SPR microscopy images and immunofluorescence images of the same cell
Figure 2. SPR microscopy images and immunofluorescence images of the same cell
Creative Biolabs also provides membrane protein study using an artificial bilayer to keep membrane proteins in their native structures. Compared with ex situ, SPR microscopy enables the direct in situ detection of ligand-receptor interaction more accurate. Therefore, SPR microscopy shows great potential in the discovery of drugs, including monoclonal antibodies and small-molecule drugs that target membrane proteins.
References
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.