Aptamers are a class of potential targeted therapeutic drugs. For applications requiring long-term systemic administration, aptamers must achieve high-affinity target binding, maintain high-stability, tolerability and ease of chemical synthesis in vivo. Therefore, we propose the development service of 2' O-methyl (OCH3) modified aptamers, which can improve the stability of aptamers without affecting the affinity.
Background of 2' O-methyl (OCH3) Modified Aptamer
Aptamers are oligonucleotides that interact highly specifically with molecular targets such as proteins, similar to antibodies. Aptamers can be made into specific and selective interactions, and often inhibit the function of protein targets. There is considerable interest in the use of aptamers as therapeutic drugs in a manner similar to therapeutic antibodies. In order to make aptamers suitable for use as therapeutic drugs, it must be synthesized cheaply, safe and stable in vivo. Wild-type RNA and DNA aptamers are unstable in vivo because they are easily degraded by nuclease. Some studies have shown that the ability of anti-nuclease degradation can be significantly improved by adding modification groups at nucleotide 2 position. In addition to 2’-fluoro and 2'-amino modifications, 2' O-methyl modified aptamers are resistant to nuclease, and have attracted a lot of attention because of their low cost and no obvious safety problems in circulating into host DNA.
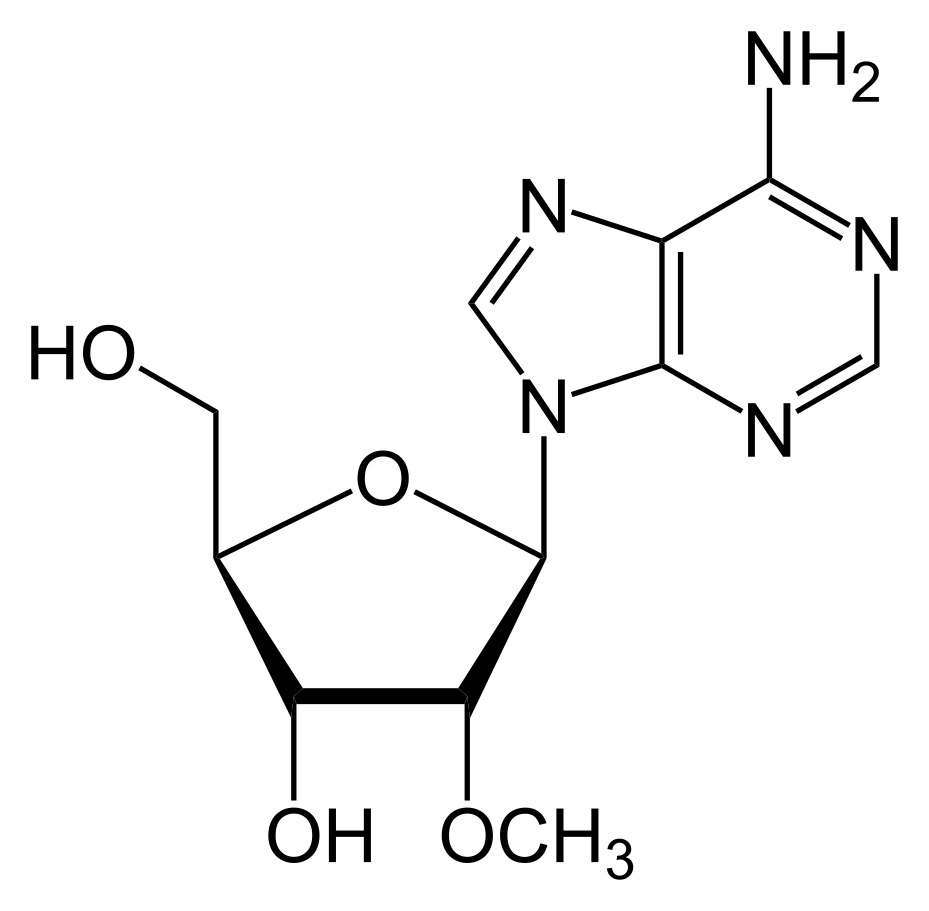 Fig 1 2' O-methyl modification.1
Fig 1 2' O-methyl modification.1
2' O-methyl (OCH3) Modified Aptamer Development in Creative Biolabs
In addition to the wide application prospect in the medical field, another attractive feature of aptamers is the easy-to-introduce chemical functions, which enable aptamers to have the versatility required for a wide range of applications. The process of introducing chemical modifications to enhance the stability of anti-nuclease is specifically: after selecting aptamers by systematic evolution of ligands by exponential enrichment (SELEX) using natural DNA/RNA, structure-activity relationship (SAR) studies are performed to develop functional aptamers. This includes system truncation and synthesis of aptamer analogues, and then studying the affinity of each analogue to determine the shortest aptamer length and the highest affinity. The truncated aptamers with the highest affinity then systematically replace the modified anti-nucleic acid analogs at the 3' and 5' ends to prevent the attack of exonuclease.
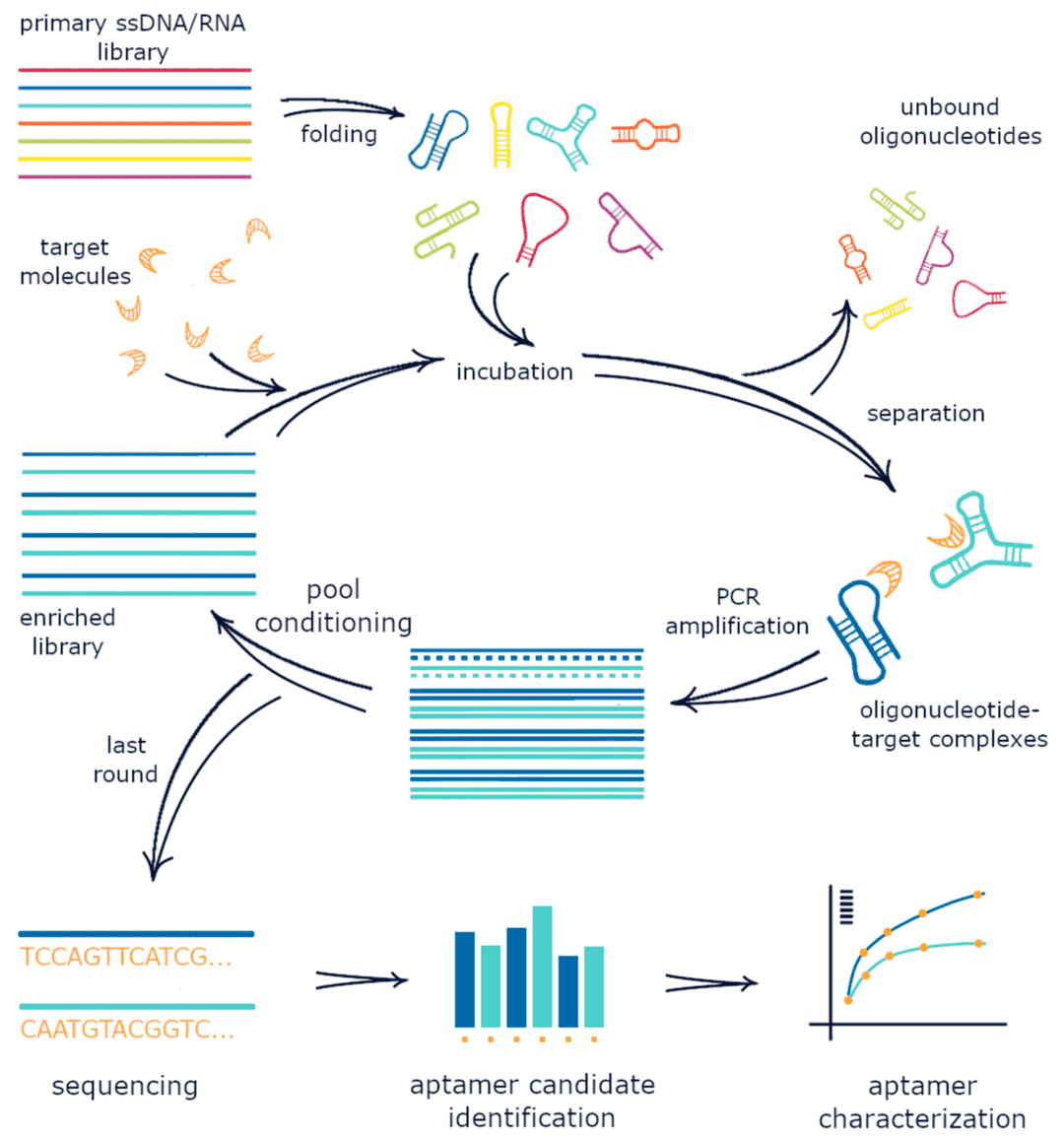 Fig. 2 Schematic representation of the SELEX process.2, 3
Fig. 2 Schematic representation of the SELEX process.2, 3
What Can We Do?
A large number of studies have shown that one way to avoid nuclease instability is to use chemically modified and stable 2'- O-x-analogues at the 3' and 5' ends to produce the second generation of functional DNA aptamers. At Creative Biolabs, we provide a systematic aptamer modification service to better meet the needs of researchers. If you are interested in our service, please contact us for your exclusive solution.
References
-
By Yikrazuul (talk) - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=28399390.
-
Komarova, Natalia, and Alexander Kuznetsov. "Inside the black box: what makes SELEX better?" Molecules 24.19 (2019): 3598
-
under Open Access license CC BY 4.0, without modification
Related Product
Questions & Answer
A: 2' OCH3 modification involves the replacement of the 2'-OH group of the nucleotide with a methoxy group. 2' OCH3 is a common sugar modification that can be used to generate aptamers with high nuclease resistance.
A: Target-specific aptamers are developed from oligonucleotide libraries modified with 2' OCH3 adenine and guanine by electrophoretic mobility shift analysis. The inhibition and stability of the candidate modified aptamers are determined, and their affinity is also measured. Finally, 2' OCH3 pyrimidine-containing aptamers for a variety of protein targets are screened by the SELEX process.
A: The affinity is measured through nitrocellulose filter binding assay. We can select target-specific, high-affinity 2'OCH3-modified aptamers by this method.
For Research Use Only.
Related Sections:

 Fig 1 2' O-methyl modification.1
Fig 1 2' O-methyl modification.1
 Fig. 2 Schematic representation of the SELEX process.2, 3
Fig. 2 Schematic representation of the SELEX process.2, 3