Product List Background Factor P Functional Service
Background
Complement factor P, also known as Properdin or CFP, is a 53 kDa glycoprotein that is a key positive regulator of the complement activity. It is produced primarily by leucocytes rather than hepatocytes. It circulates in plasma in the form of cyclic oligomers of head-to-tail associations of monomers, such as dimers, trimers, and tetramers, among which the tetramer is the prevalent form. Each monomer contains six thrombospondin type 1 repeat domains (TSR1-6) and a truncated N-terminal domain (TSR0).
Properdin binds to and stabilizes the C3 and C5 convertase enzyme complexes, promoting alternative pathway activation. It also can increase the formation of the alternative pathway C3 convertase by increasing binding of factor B to P, C3b complexes to promoting activation of the alternative pathway. Besides, properdin is also involved in phagocytosis. Properdin, binding to early apoptotic T cells, promotes the phagocytic uptake of the apoptotic cells by macrophages and dendritic cells. Furthermore, properdin is also indicated to suppress the degradation of the complement C3 beta chain (C3b) mediated by CFI-CFH. Deficiency of properdin leads to impaired alternative pathway function, thereby triggering various diseases such as meningococcal infections, arthritis, asthma, and kidney and cardiovascular diseases.
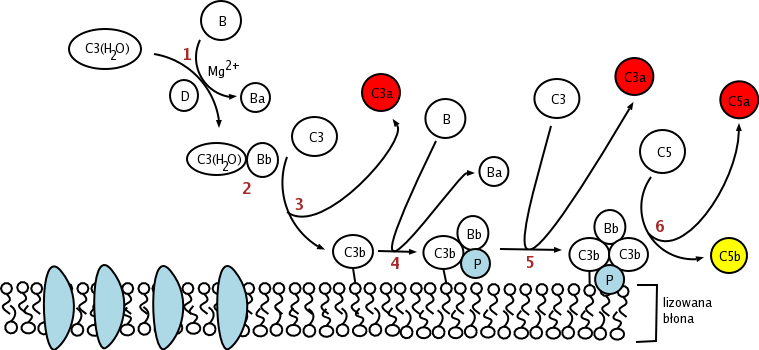 Fig.1 The functions of complement Factor P in the alternative complement pathway. Distributed under CC BY-SA 3.0, from Wiki, without modification.
Fig.1 The functions of complement Factor P in the alternative complement pathway. Distributed under CC BY-SA 3.0, from Wiki, without modification.
Factor P Functional Service
Creative Biolabs provides a comprehensive range of Factor P-related products, encompassing anti-Factor P monoclonal/polyclonal Antibodies, complement Factor P ELISA Assay kits, and complement Factor P Proteins, which are ideal for research, diagnostics, and therapeutic development. These products offer high specificity, sensitivity, and reproducibility, enabling accurate and reliable detection and quantification of Factor P.
Complement Factor P functional testing assesses the biological activity of Factor P in a blood sample, offering valuable information about its involvement in both normal physiological functions and disease-related processes. This testing can be performed using a variety of methods, typically ELISA, as well as Western blotting, hemolytic assays, and complement activation assays.
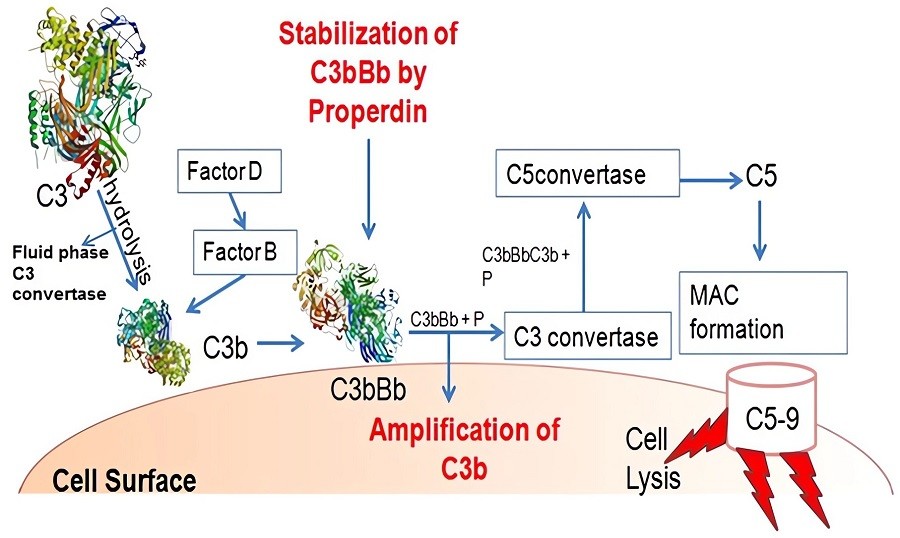 Fig.2 Properdin/Factor P can be found in serum in different forms.1
Fig.2 Properdin/Factor P can be found in serum in different forms.1
Our Factor P-related products can be employed in these assays to study Factor P's involvement in complement activation, inflammation, and immune response. Researchers have utilized these products to investigate the role of Factor P in age-related macular degeneration and other eye disorders. In a study, Factor P testing has been used to elucidate its influence on the regulation of the alternative complement pathway, leading to new insights into its potential therapeutic implications for complement-related disorders.
Creative Biolabs provides Factor P-functional services, including customized assay development, technical support for experimental design, and in-depth analysis of Factor P activity. Our services help customers accelerate research with precision, offering expert guidance and high-quality, tailored solutions to meet their specific needs.
Reference
-
Kouser, Lubna, et al. "Properdin and factor h: opposing players on the alternative complement pathway “see-saw”." Frontiers in immunology 4 (2013): 93. Distributed under Open Access license CC BY 3.0. The image was modified by extracting and using only part of the original image.


 Datasheet
Datasheet Fig.1 The functions of complement Factor P in the alternative complement pathway.
Fig.1 The functions of complement Factor P in the alternative complement pathway. Fig.2 Properdin/Factor P can be found in serum in different forms.1
Fig.2 Properdin/Factor P can be found in serum in different forms.1
