Complement cascades are under strict regulation in our immune responses to avoid attacking self-components. With advanced technologies and long-term scientific expertise, Creative Biolabs has applied a series of regulator development services for numerous complement components. We are proud to offer high-quality and custom services to help you get milestone success. Now, we have successfully developed a wide range of complement regulators products (such as factor I) to meet our clients’ demands precisely.
Introduction of Factor I
As a key part of innate and adaptive immune responses, complement system participates in the elimination of pathogens and altered host cells, maintaining the homeostasis of our body. Complement regulation is critical for the prevention and control of the disease.
Factor I (FI), also known as C3b/C4b inactivator, is a serine proteinase that is essential for regulating the complement cascade. FI usually presents a proteolytically inactive form, suggesting that it circulates in a zymogen-like state despite being fully processed to the mature sequence. In the alternative pathway (AP), FI interacts with its cofactor-FH to mediate the proteolytic inactivation of the central complement protein C3b to form iC3b.
Structure of Factor I
FI is a glycoprotein heterodimer consisting of a disulfide-linked heavy chain and light chain. The heavy chain comprises four domains, namely a FI membrane attack complex (FIMAC) domain, SRCR (scavenger receptor cysteine-rich) domain (also known as a CD5 domain), and low-density lipoprotein receptor 1 and 2 (LDLr1 and LDLr2) domains. It is documented that the heavy chain is responsible for the enzyme inactive form. When the complex consisting of C3b or C4b, a cofactor protein (FH, C4BP, CR1, and MCP) binds to the heavy chain of FI, the enzyme is fully activated by allostery. The light chain of FI contains only the serine protease domain, and its catalytic triad (His-362, Asp-411, and Ser-507) is responsible for specific cleavage of C3b and C4b.
Functions and Clinical Significance of Factor I
FI can cleave and inactivate the complement components C4b and C3b, and it prevents the assembly of the C3 and C5 convertase enzymes, decreasing the activation of the complement system. FI deficiency will subsequently lead to low levels of C3, factor B, factor H, and properdin. FI dysregulation or deficiency is closely associated with various clinical implications.
-
Recurrent Infections
FI deficiency is associated with increased susceptibility to infection, including infections of the upper respiratory tract, ears, skin, and urinary tract, which may also contract more serious infections such as pneumonia, meningitis, and sepsis, which may be life-threatening.
-
Age-Related Macular Degeneration (AMD)
It is reported that mutations in the CFI gene contribute to the development of AMD, which is may be caused by the dysregulation of the alternative pathway, leading to increased inflammation in the eye.
-
Atypical Hemolytic Uremic Syndrome (aHUS)
FI deficiency or heterozygous mutations will lead to complement overactivation, which is one of the main causes of aHUS.
Owing to the vital roles in complement activation and immune modulation, FI has become a promising and effective target for the treatment of diseases associated with abnormal or loss of complement control.
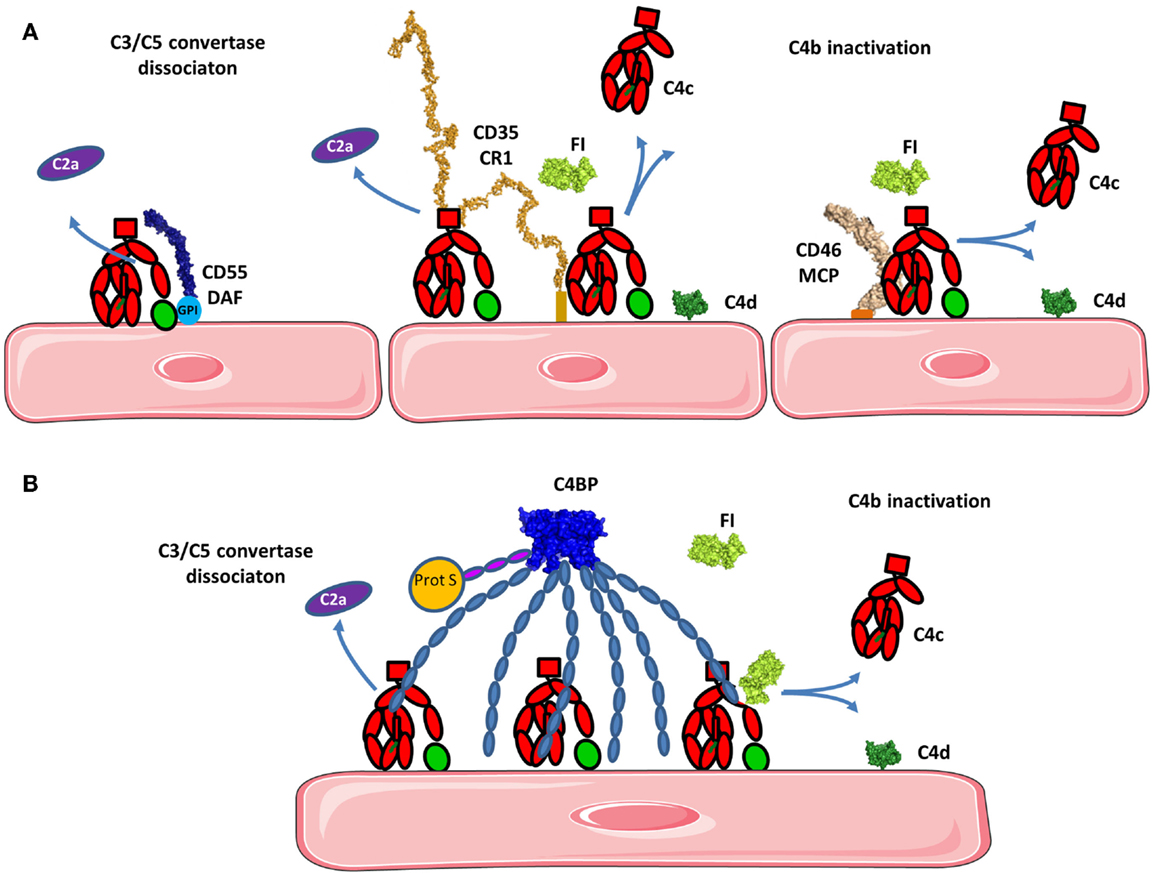
Fig. 1 Schematic of complement activation.1, 2
Why Choose Us?
Creative Biolabs can offer a full range of formulation development services for complement products and therapeutic drug exploration to meet your particular demands precisely.
-
Industry leadership
Our industry-leading expertise in the biopharma research and scalable formulation can help you promote your therapeutics and medical projects.
-
Professional team and abundant experience
We have successfully completed a lot of drug discovery cases, accumulating rich experience. Our scientists are specialized in regulatory knowledge and scientific applications specific to drug development.
-
Cost effective and after-sale service
For more information about our complement services, please contact us now.
References
-
Merle, Nicolas S., et al. "Complement system part I–molecular mechanisms of activation and regulation." Frontiers in immunology 6 (2015): 262.
-
under Open Access license CC BY 4.0, without modification.
Related Product
For Research Use Only.
Related Sections:

