Creative Biolabs has over ten years of experience in Microfluidic technology and infectious disease diagnosis. Based on vast experience and solid technical strength, our focus is to exceed your expectations while providing accurate and reliable services for our global customers.
Infectious diseases, such as HIV/AIDS, malaria, sepsis, and tuberculosis, are leading causes of mortality and morbidity in developing countries. Due to inadequacies in accessing adequate health care infrastructure, over 95% of deaths from infectious diseases are caused by a lack of proper diagnostics and treatments. Therefore, infectious diseases caused by viruses, parasites, and bacteria have been identified as the top priority in developing cost-effective diagnostic methods.
Infectious Diseases Diagnosis with Microfluidic
Microfluidic platforms enable medical solutions for infectious disease diagnosis and real-time monitoring more rapidly and accurately and analyze diverse clinical samples, including blood, oral fluid/saliva, and urine. Over the past decade, research efforts in Microfluidics have been made to develop methods that can facilitate low-cost diagnosis of infectious diseases, especially in resource-poor settings. For example, some researchers have developed a simple and mass-producible Microfluidic platform using multiplexed digital RT-PCR to quantify HIV viral load in patients' samples for HIV/AIDS diagnostic and monitorization. Besides, a Microfluidic system can be used to detect the HIV integrase gene by LAMP reaction, which also shows a positive outcome for the HIV diagnosis.
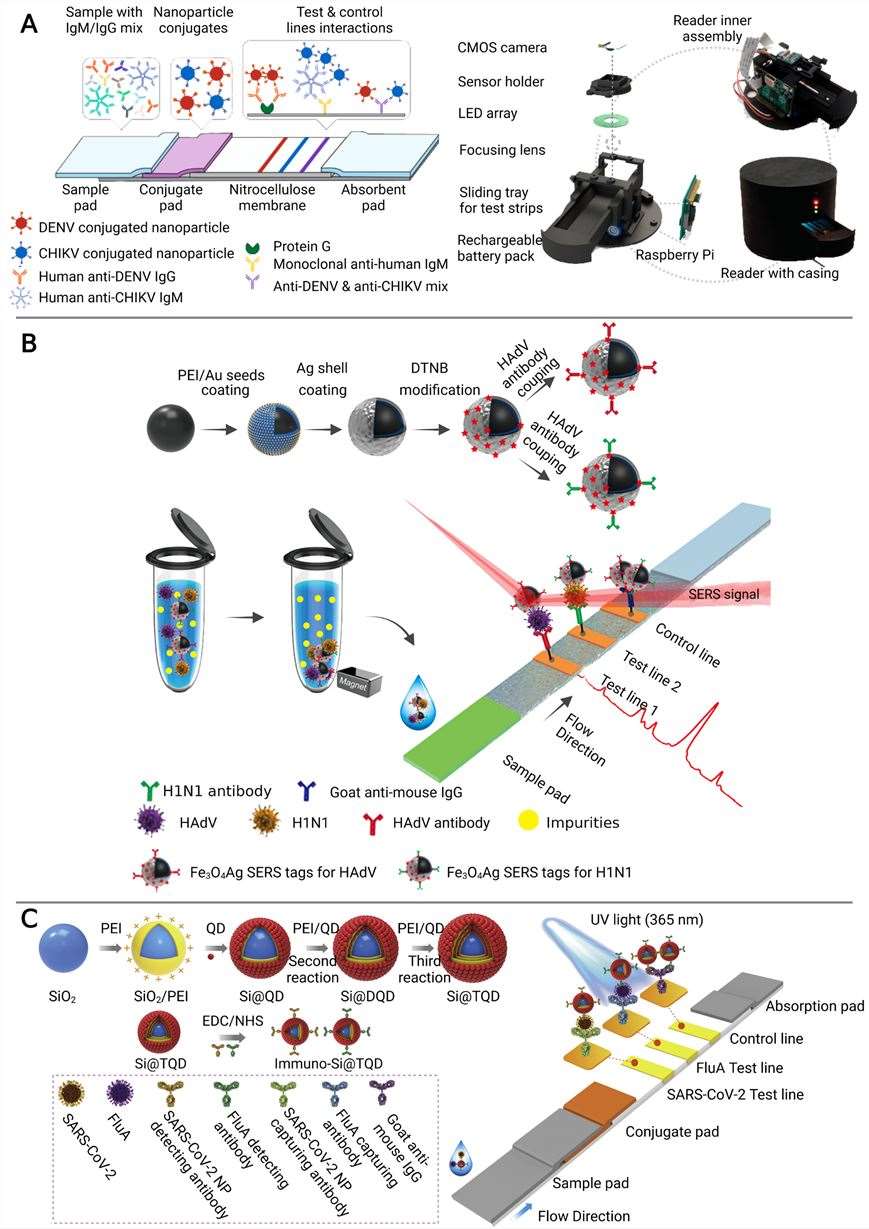 Fig.1 Multiplexed detection of infectious disease biomarkers utilizing microfluidic immunosensor platforms.1, 2
Fig.1 Multiplexed detection of infectious disease biomarkers utilizing microfluidic immunosensor platforms.1, 2
Advantages of Microfluidic-based Diagnostics
Microfluidic-based diagnostics have significant advantages over conventional diagnostics for detecting infectious diseases, such as cost-effectiveness, ease of use, reduced sample consumption, increased portability, and disposability. In addition, various on-chip diagnostic modules have been integrated within the same fluidic chips, providing excellent flexibility and functionality. Identifying pathogenic microorganisms using Microfluidic is also shown great promise, mostly because the sample preparation procedure is dramatically reduced. The pathogen can be directly detected, translating into increased speed and accuracy. Therefore, Microfluidics could be an extremely attractive platform for providing infectious disease diagnostics for the developing world.
Our Services for Infectious Diseases Diagnosis
Microfluidic technologies make the development of fast, sensitive, and portable diagnostic tools possible, thus promising rapid and accurate diagnosis and monitoring of infectious diseases. Creative Biolabs is a global provider to offer the integrated Microfluidic-based platform for infectious disease diagnosis for our global customers. Our Microfluidic technology presents itself as a flexible detection platform that can be readily adapted to specific infectious pathogen-related needs. Creative Biolabs offers custom diagnosis services for infectious diseases as follows:
- Nucleic acid detection based on Microfluidics
- Protein detection based on Microfluidics
- Integrated approach for multiplexed protein and DNA analysis
- Single cell analysis based on Microfluidics
With our extensive experience and advanced platforms, Creative Biolabs is confident in offering the best infectious disease diagnosis services for our customers worldwide. If you are interested in our services, please do not hesitate to contact us for more details.
Published Data
1. Microfluidic Affinity Capture Assay for Ultrasensitive Intact SARS-Cov-2 Particles Detection in Complex Biofluids
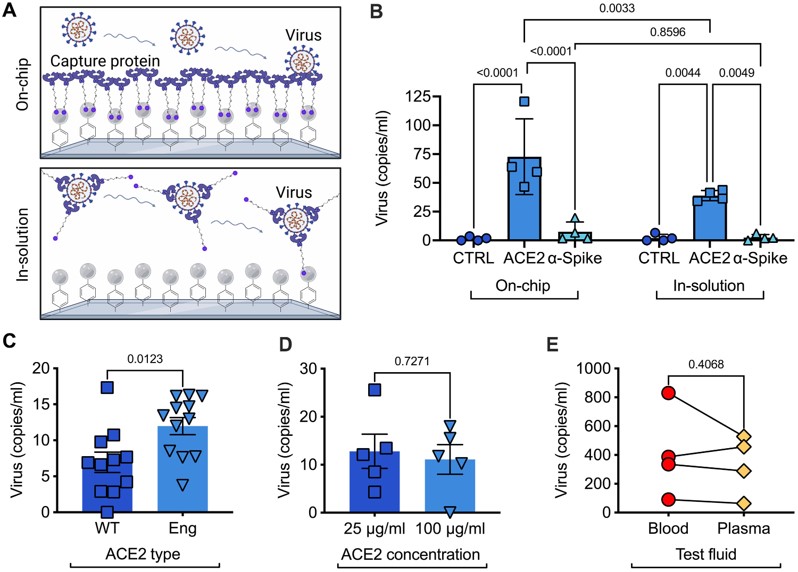 Fig.2 Microfluidic affinity capture for viral diagnostic.3,2
Fig.2 Microfluidic affinity capture for viral diagnostic.3,2
This study developed an affinity-based microfluidic device for specifically capturing intact SARS-CoV-2 from biofluids, addressing the challenge of confounding biomolecules coisolated with viral nucleic acids. The device used engineered angiotensin-converting enzyme 2 to selectively capture the virus from plasma and others. It incorporated a staggered herringbone pattern, nanoparticle coating on the surface, and refined processing conditions, reaching a limit of detection of 3 viral copies per milliliter. The assay was validated on 103 plasma, 29 stool, and 36 saliva samples from COVID-19 patients, detecting SARS-CoV-2 in 72% of plasma samples. Longitudinal monitoring demonstrated its ability to detect active infections. This technology can be applied to other viruses, offering broad potential for viral load monitoring and disease management.
2. Multiplexed CRISPR Microfluidics for Respiratory Virus Testing and SARS-CoV-2 Variant Identification
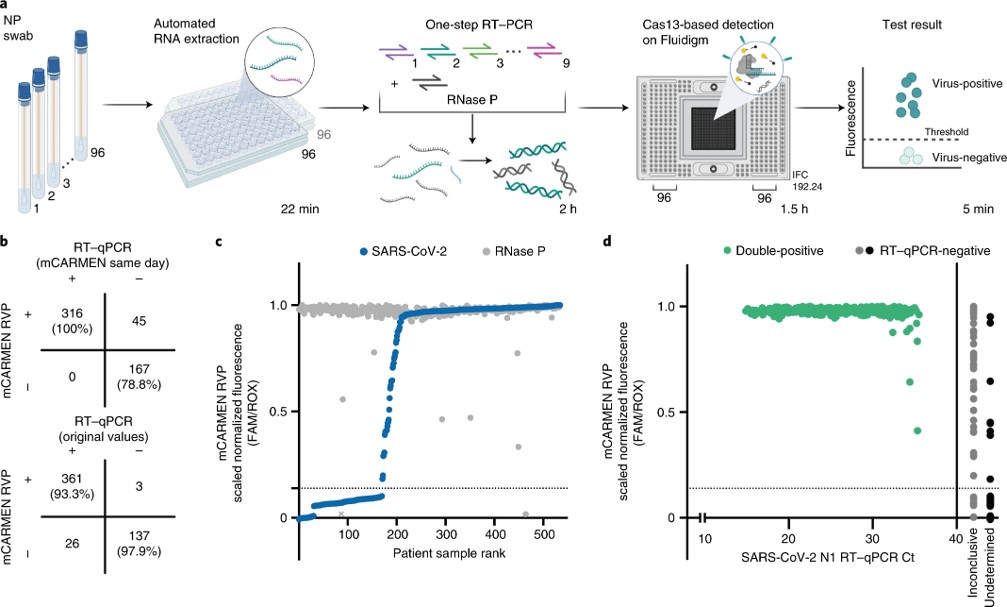 Fig.3 Workflow of mCARMEN.4,2
Fig.3 Workflow of mCARMEN.4,2
This study introduced the microfluidic Combinatorial Arrayed Reactions for Multiplexed Evaluation of Nucleic acids (mCARMEN), a cost-effective platform combining gene-editing-based diagnostics with microfluidics for clinical use. Researchers developed the mCARMEN respiratory virus panel to detect up to 21 viruses, including SARS-CoV-2, other coronaviruses, and influenza strains, and validated its performance on 525 patient specimens in an academic setting and 166 in a clinical setting. They also created a panel to identify 6 SARS-CoV-2 variants, achieving near-perfect concordance with sequencing in 2,088 patient specimens. Additionally, a combined Cas13 and Cas12 approach enabled quantitative viral measurement. The mCARMEN platform enables multiple virus and variant surveillance simultaneously for rapid SARS-CoV-2 variant detection.
References
- Chen, Fumin, et al. "Multiplex detection of infectious diseases on microfluidic platforms." Biosensors 13.3 (2023): 410.
- Distributed under Open Access license CC BY 4.0, without modification.
- Rabe, Daniel C., et al. "Ultrasensitive detection of intact SARS-CoV-2 particles in complex biofluids using microfluidic affinity capture." Science Advances 11.2 (2025): eadh1167.
- Welch, Nicole L., et al. "Multiplexed CRISPR-based microfluidic platform for clinical testing of respiratory viruses and identification of SARS-CoV-2 variants." Nature medicine 28.5 (2022): 1083-1094.
For Research Use Only.