Creative Biolabs is a well-recognized leader in the field of the custom in vitro diagnostic (IVD) antibody discovery and development, as well as IVD assay development. We are dedicated to the development of innovative IVD reagents with higher specificity and sensitivity, to help increase the accuracy of the clinical diagnosis and to support clinicians in the effective management of different types of mucous membrane pemphigoid (MMP).
Introduction to MMP
Mucous membrane pemphigoid (MMP) has been known by different names including cicatricial pemphigoid (CP), benign mucous membrane pemphigoid, benign mucosal pemphigoid, ocular pemphigus, and scarring pemphigoid. MMP is the subgroup of pemphigoid, a heterogeneous group of rare chronic autoimmune subepithelial blistering, which primarily affects various mucous membranes of the body and occasionally the skin with blistering lesions. In vivo, it is characterized by linear deposition of IgG, IgA, or C3 along the epithelial basement membrane zone (BMZ). The mucous membranes of the mouth and eyes are most often affected and the mucous membranes of the nose, throat, genitalia, and anus may also be affected.
Diagnostic Tools of MMP
Usually, by using routine histopathology diagnosis, MMP can be differentiated from other mucocutaneous diseases, such as lichen planus, erythema multiforme, and pemphigus vulgaris. However, it’s hard to differentiate MMP from other subepithelial autoimmune diseases depends upon neither routine histopathology nor immunopathology. The diagnosis of MMP is often delayed by the non-specific presentations in the early stage or inconclusive biopsies. To solve the diagnosis dilemmas, the differential diagnosis must be made on the basis of a combination of clinical and histopathological features. MMP is diagnosed based on a thorough clinical evaluation, a detailed patient history, identification of characteristic findings and certain tests known as a biopsy, direct immunofluorescence (DIF), and indirect immunofluorescence (IIF). For a biopsy, a small sample of skin tissue is removed (biopsy) and microscopically examined. For DIF, a second biopsied skin sample is tested to detect the presence of the specific autoantibodies (e.g. IgA, IgG, and C3) at the BMZ. To identify circulating autoantibodies to the BMZ, IIF with normal human skin as the substrate is usually performed. Besides, serological analyses detecting circulating autoantibodies are also included in DIF-negative or DIF-unavailable cases.
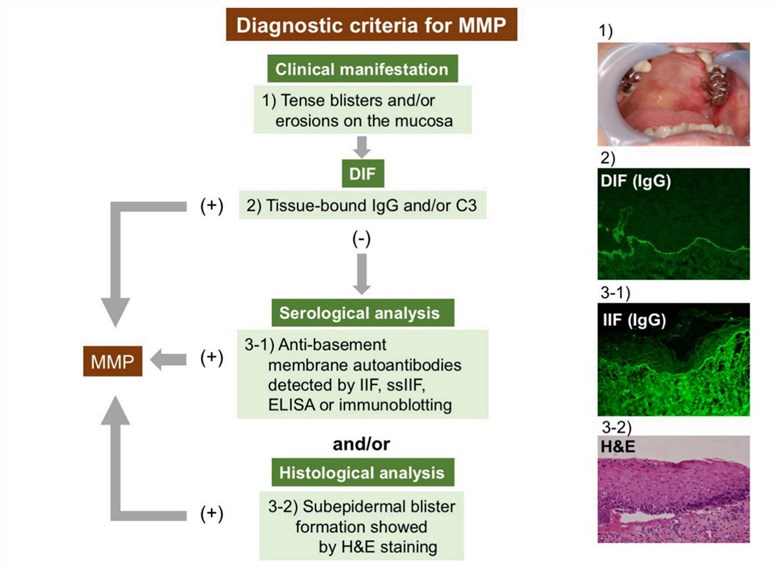 Fig.1 Diagnostic strategy for MMP.1
Fig.1 Diagnostic strategy for MMP.1
IVD Immunoassay & Kit Development Services Provided by Creative Biolabs
Based on the specific recognition between one or more antibodies and an antigen, immunoassays allow the detection and quantification of various antigens and/or antibodies in different types of samples (serum, plasma, urine, saliva, environmental media, etc.). Diagnostic immunoassays that are developed for different biomarkers support the early diagnosis of a wide range of diseases, including MMP. With extensive experience in the IVD field, Creative Biolabs offers custom assay design, development, verification, validation, and manufacturing of your product. Our capabilities include antigen production, antibody production and characterization, development of immunoassays, and manufacturing. Our professional project management team assists you throughout the entire course of a project. Specifically, Creative Biolabs offers comprehensive contract development services including:
- IVD Antibody Development
- Antibody Pair Development
- Antibody & Protein Conjugation
- IVD Immunoassay Development
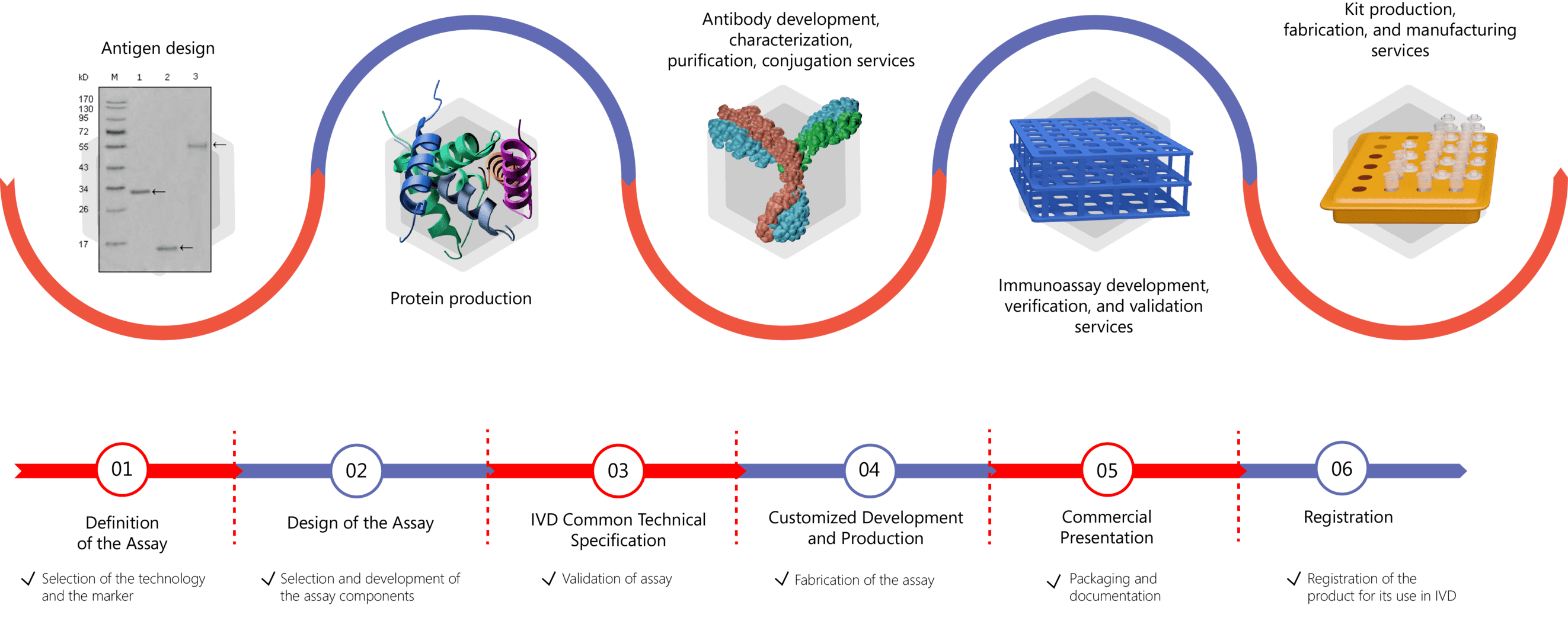
Our services can be tailored targeting different serological or tissue biomarkers as follows:
Features of Our Services
- End-to-end assay lifecycle services
- Fully committed to building mutually beneficial long-term relationships
- Fully customized and adapted to your specific needs
- Years of expert experience with all types of technology on any type of analyzer
Creative Biolabs is happy to offer you our expertise to develop a diagnostic tool according to your specifications. Please feel free to contact us for more information and discuss your project needs.
Reference
- Kamaguchi, Mayumi, and Hiroaki Iwata. "The diagnosis and blistering mechanisms of mucous membrane pemphigoid." Frontiers in Immunology 10 (2019): 34. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.