Cancer is a class of diseases characterized by out-of-control cell growth with the potential to invade or spread to other parts of the body. The unregulated growth of cells leads to the formation of tumors, which can be benign or malignant. Tumors that stay in one spot and demonstrate limited growth are generally considered to be benign, while cells that can pass more easily through smaller gaps, invade nearby tissues, and spread to other parts of the body, are malignant. Cancer is a genetic disease which is caused by changes to genes that control the way our cells function, especially how they grow and divide. The genes involved include oncogenes, tumor suppressor genes, suicide genes, and DNA-repair genes. Environmental risk factors that contribute to cancers include tobacco use, obesity, poor diet, lack of physical activity, excessive alcohol, infections, etc. Different treatment options exist for cancer treatment, such as chemotherapy, radiation, surgery, immunotherapy, and laser therapy, but the choices largely depend on the type, location, and grade of cancer.
Diagnosis of Cancer
Diagnosis of cancer is often made using a combination of different tests. Firstly, cancer screening, e.g., blood or urine tests, DNA tests, and medical imaging, can be conducted to detect cancer before symptoms appear. Secondly, people with suspected cancer symptoms can undergo imaging scans, such as CT, ultrasound, and magnetic resonance imaging (MRI) scan to identify cancerous lesions in the body. Thirdly, the tissue biopsy that indicates the type of cell that is proliferating, its histological grade, genetic abnormalities, and other features can be conducted to determine a definitive diagnosis. Besides, other types of tissue tests such as cytogenetics and immunohistochemistry can be performed to obtain information about molecular changes (such as mutations, fusion genes, and numerical chromosome changes).
Molecular Biomarkers of Cancer
Molecular biomarkers, that are present in the blood, other body fluids, or tissues, are altered during tumor progression. The use of these biomarkers is of great value in (i) screening and early detection of cancer, (ii) aid in the diagnosis of cancer, (iii) determine response to therapy, (iv) prognostic indicator of disease progression, and (v) indicate relapse during the follow-up period. The employment of high-throughput techniques has allowed the identification of a number of molecular biomarkers of may cancer types. For instance, carcinoembryonic antigen (CEA) is a glycoprotein produced by many different neoplasms and is a highly sensitive biomarker (60-90%) for colorectal carcinoma. Prostate-specific antigen (PSA) and prostate-specific membrane antigen (PSMA) are useful biomarkers for the detection of prostate cancer. Besides, circulating CA125 antigen is recommended for clinical use in the US for ovarian cancer screening of high-risk women with ovaries. Due to limited sensitivity and specificity of a single marker, the detection of a panel of biomarkers simultaneously has been suggested and investigated for improved performance. Further, with the development of technologies, more and more molecules have been identified as novel potential biomarkers.
The Use of In Vitro Diagnostic (IVD) Immunoassays for Biomarker Detection
Different techniques have been developed for the detection or quantification of different types of disease biomarkers. Among them, immunoassays continue to be the most sensitive, specific, and selective technology to interrogate protein-based markers. These immunoassays use antibodies directed against the protein of interest to quantitatively measure or qualitatively detect the antigens present in all kinds of samples. The most widely used immunoassay methods include western blot, ELISA (enzyme-linked immune-sorbent assay), LFIA (lateral flow immunoassay), CLIA (chemiluminescent immunoassay), immunohistochemistry (IHC), etc. Development of antibody-based assays is a time-consuming, resource-intensive effort. The use of high-quality antibodies with high specificity and sensitivity is of great importance for this purpose.
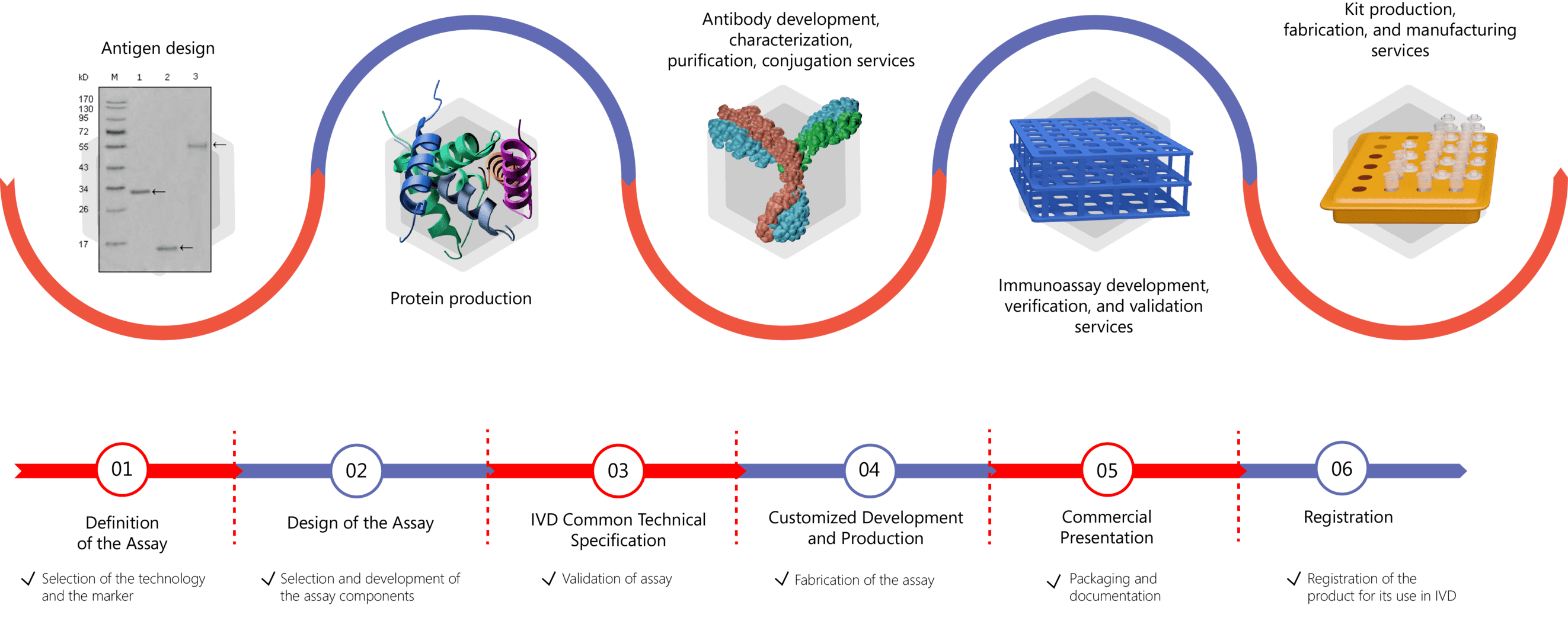
IVD Antibody & Immunoassay Development Services Provided by Creative Biolabs
Creative Biolabs offers our customers its long years of valuable experience in the development of assays and kits for diagnostic and research purposes. Our customized IVD immunoassay development service can cover different stages within the scope of the assay, ranging from antibody generation, antibody conjugation and immobilization, protein production and purification, assay development, verification, validation, and kit production. For more information, please click the following links:
- IVD Antibody Development
- Antibody Pair Development
- Antibody & Protein Conjugation
- IVD Immunoassay Development
Our expertise lies in a wide range of cancer types and a wide spectrum of biomarkers as follows:
| Follicular Adenoma › | Melanoma › | Leukemia › |
Testicular Cancer ›
Chorionic Gonadotropin Alfa-fetoprotein Lactate Dehydrogenase
|
Breast Cancer › |
| Pancreatic Cancer › | Bladder Cancer › | Brain Cancer › | Gastrointestinal Cancer › | Prostate Cancer › |
| Cholangiocarcinoma › | Germ cell cancer › | Liver Cancer › | Neuroendocrine Tumor › | Renal Cancer › |
| Colorectal Cancer › | Glioblastoma › | Lung Cancer › | Myeloma › | Esophageal Cancer › |
| Glioma › | Lymph Cancer › | Ovarian Cancer › | Thyroid Cancer › | Others |
Features of Our IVD Antibody Development Services
- Our repertoire includes both established and novel biomarkers
- A specialized technical team and abundant experience in antibody development
- State-of-the-art techniques and short turnaround time
- Services are tailored to meet the needs of our valued IVD clients.
In addition, Creative Biolabs provides one-stop diagnostic immunoassay development services, covering feasibility analysis, assay design, assay protocol establishment, validation, and production. Please feel free to contact us for more information and a formal quote.
References
- Sethi, S., (2013). “Clinical advances in molecular biomarkers for cancer diagnosis and therapy.” International journal of molecular sciences, 14(7), 14771-14784.
- Qoronfleh, M. W., (2010). “Protein biomarker immunoassays opportunities and challenges.” Drug Discovery World, 11(1), 19-28.
- Nagpal, M., (2016). “Tumor markers: A diagnostic tool.” National journal of maxillofacial surgery, 7(1), 17.
For Research Use Only.