Chemiluminescent immunoassay (CLIA), a sophisticated variant of the enzyme immunoassay (EIA), serves as a key biochemical technique in immunology and a diagnostic tool across various industries. Creative Biolabs excels in developing ELISA-based kits, offering numerous assays for specific disease-related protein detection. We've optimized enzyme-labeled methods for CLIA, providing high-quality, cost-effective testing services to ensure project success.
How Does It Work?
CLIA operates on a fundamental immunological principle: the specific binding of an antigen to its matched antibody. This approach uses an enzyme to accurately identify either the antigen or the antibody. When this enzyme-labeled complex reacts with a specific substrate, it triggers a chemical reaction that produces photons of light, rather than a color change, as seen in traditional ELISA. This emission of light, known as luminescence, occurs as the excited product of the chemical reaction returns to a more stable ground state. The intensity of the emitted light is then precisely measured by a luminometer. The brilliance of this method lies in its ability to directly correlate the observed luminescence with the quantity of the specific biomolecule present in the sample, making it an exceptionally accurate and sensitive detection system for targets like peptides, proteins, and hormones in fluid samples.
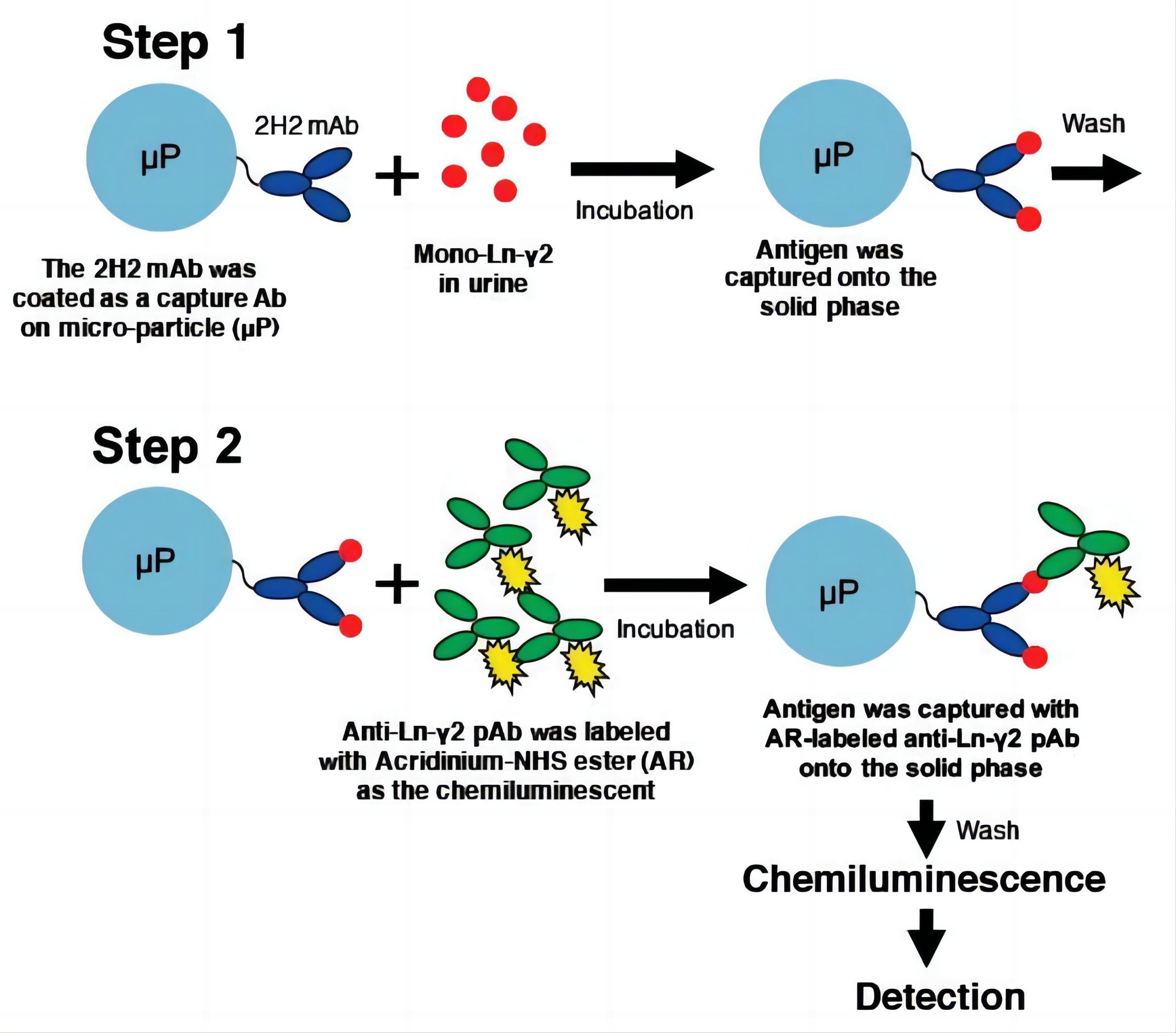 Fig.1 The principle of CLIA.1
Fig.1 The principle of CLIA.1
Advantages of Our CLIA Platform
Creative Biolabs is dedicated to pushing the boundaries of CLIA technology, consistently developing highly sensitive, flexible, quantitative, and user-friendly immunoassay kits. Our commitment to innovation yields significant advantages:
Ultra-Sensitivity: CLIA kits enable the detection of minute quantities of biological molecules, crucial for early diagnosis and low-concentration biomarker monitoring.
Wide Dynamic Range: A broad linear relationship between luminous intensity and target substance concentration is achieved, allowing accurate quantification across a vast range of analyte levels.
Enhanced Reactions: Through meticulous optimization, assays incorporate advanced enhancers to amplify chemiluminescent signals, further boosting detection limits and improving performance.
Rapid Efficiency: Unlike some traditional methods, CLIA assays often do not require stop reactions and feature significantly shorter incubation times, streamlining laboratory workflows and accelerating results.
Our CLIA Based Kit Development Services
At Creative Biolabs, we don't offer generic solutions; we provide highly customized CLIA-based kit development services meticulously tailored to your unique diagnostic needs. Our comprehensive service encompasses every stage, from initial concept validation and strategic assay design to rigorous validation and scalable manufacturing. We specialize in optimizing antigen and antibody selection, precisely conjugating luminophores, and fine-tuning every reaction parameter to ensure unparalleled assay performance. Whether you require a novel diagnostic assay for a specific biomarker, an enhanced version of an existing test, or a high-throughput screening solution, our expert team collaborates closely with you to engineer a CLIA kit that meets your exact specifications and delivers robust, reliable results. We are dedicated to translating your scientific vision into a high-quality, practical diagnostic product.
Applications
Clinical Diagnostics
The CLIA kits are pivotal in clinical settings for speedy and reliable identification of numerous illnesses. This includes early detection and monitoring of infectious diseases like Hepatitis B/C, HIV, and COVID-19, precise quantification of tumor markers such as PSA, CEA, and AFP for cancer screening and progression monitoring, and highly sensitive measurement of hormone levels (e.g., thyroid hormones, reproductive hormones) for endocrine disorders and fertility assessment.
Food Safety Testing
In the food industry, CLIA assays are essential for detecting contaminants and allergens at very low levels. This includes screening for bacterial toxins (e.g., Staphylococcus aureus enterotoxins), mycotoxins in grains, and allergens like peanut proteins or gluten, ensuring consumer safety and regulatory compliance.
Environmental Monitoring
The CLIA kits provide sensitive tools for environmental analysis, enabling the detection of pollutants and hazardous substances in water and soil samples. Specific applications include monitoring pesticide residues, heavy metal ions, or certain organic pollutants, contributing to environmental protection and public health.
Research and Drug Discovery
For cutting-edge research and pharmaceutical development, CLIA offers high-throughput screening capabilities. It is widely used for quantifying drug metabolites, assessing therapeutic drug monitoring, and screening for novel biomarkers in biological samples, accelerating preclinical and clinical studies.
Published Data
1. Ultrasensitive Chemiluminescence Immunoassay for HE4 Detection Using Acridinium Ester-Labeled Antibody and GoldMag Nanoparticles
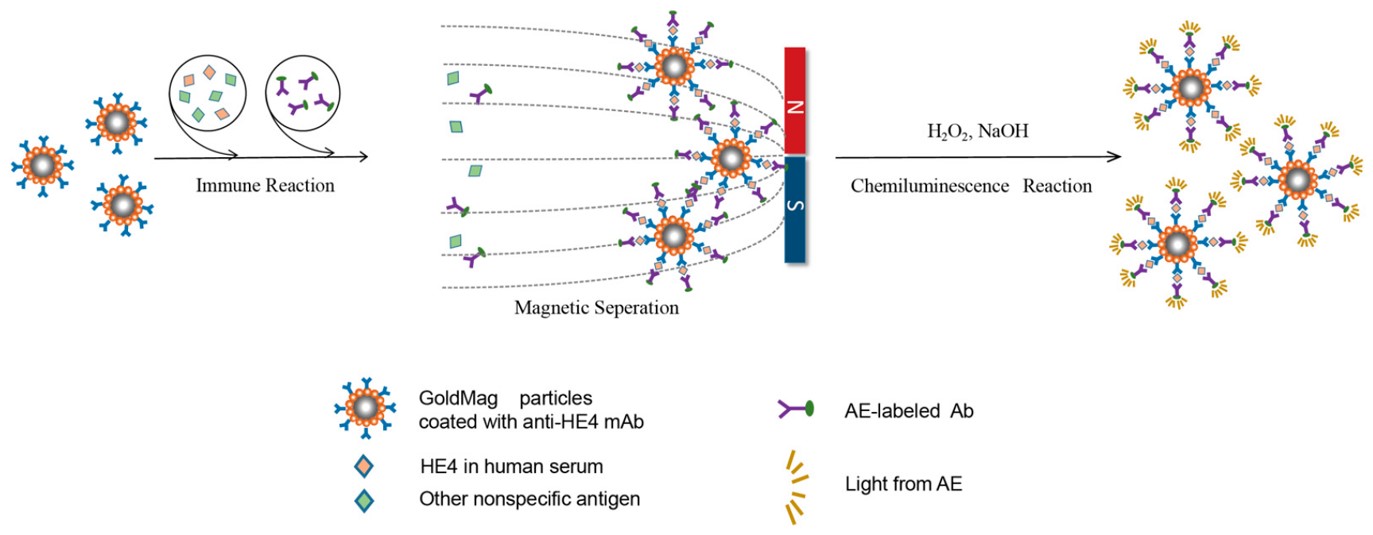 Fig.2 The scheme of the GMP-CLIA method.2,4
Fig.2 The scheme of the GMP-CLIA method.2,4
By combining the AE chemiluminescence system with GoldMag nanoparticles (GMP), researchers presented a high-sensitivity and rapid chemiluminescence immunoassay (CLIA) for detecting human epididymis protein 4 (HE4). The AE labeling of antibodies process was systematically optimized by adjusting key factors, including the AE-to-antibody molar ratio, labeling duration, and the composition of the elution buffer and trigger solution. Under optimal conditions, AE labeling achieved an average efficiency of 1.92 ± 0.08 and an antibody utilization rate of 69.77 ± 1.19%, with no loss of antibody activity. The GMP-CLIA method successfully detected HE4 within a concentration range of 0.25-50 ng·mL−1 (10-2000 pM), with a lower detection limit of 0.084 ng·mL−1 (3.36 pM). This method demonstrated sensitivity comparable to commercial kits and was successfully applied to measure HE4 in 65 human serum samples, showing strong potential for clinical diagnosis.
2. Chemiluminescence Immunoassay for Diagnosing Mushroom Poisoning
 Fig.3 Principle of MB-based CLIAs for automated detection of phallotoxins.3,4
Fig.3 Principle of MB-based CLIAs for automated detection of phallotoxins.3,4
In this study, researchers developed an ultrasensitive and automated chemiluminescence immunoassay (CLIA) using magnetic beads (MBs) for the early detection of mushroom poisoning. The method achieved limits of detection (LODs) for phallotoxins of 0.010 ng/ml in serum and 0.009 ng/ml in urine. Recovery rates ranged from 81.6% to 95.6%, with a coefficient of variation under 12.9%. The automated MB-based CLIA accurately identified Amanita phalloides samples, with results consistent with those from HPLC-MS/MS. The assay's exceptional sensitivity, reproducibility, and stability were attributed to the use of MBs as immunoaffinity carriers, chemiluminescence as a detection signal, and the complete automation of the process. This approach offers great potential for rapid clinical diagnosis of mushroom poisoning.
Service Highlights
- End-to-End Support: We offer comprehensive support from initial concept through to manufacturing and quality control, simplifying the development pipeline for our clients.
- RUO to IVD Transition: Crucially, we assist clients in navigating the complex regulatory pathway, facilitating the transfer of their tests from research use only (RUO) to in vitro diagnostics (IVD) status.
- Performance Optimization: Our meticulous assay design and validation processes ensure superior sensitivity, specificity, and a wide dynamic range, even for low-concentration analytes.
- Accelerated Development: Leveraging efficient methodologies and experienced teams, we significantly reduce development timelines, enabling faster market entry for your diagnostic solutions.
Q&A
-
Q: What makes CLIA a superior choice compared to traditional ELISA for my diagnostic kit?
A: CLIA offers significantly enhanced sensitivity and a wider dynamic range than traditional ELISA. This means it can detect lower concentrations of an analyte and quantify it accurately across a broader range of concentrations, which is critical for early disease detection or monitoring subtle changes in biomarker levels. Additionally, CLIA reactions are faster and often do not require stop reactions, leading to more efficient assay workflows.
-
Q: How do you maintain the high sensitivity of its CLIA kits?
A: Our commitment to high sensitivity begins with meticulous selection and optimization of high-affinity antibodies and antigens. We then fine-tune the enzyme-luminophore conjugation, optimize substrate concentration, and rigorously control reaction conditions. Furthermore, we employ advanced signal amplification strategies and utilize state-of-the-art luminometers to maximize photon detection, ensuring the lowest possible limits of detection.
-
Q: Can you develop a CLIA kit for a completely novel biomarker?
A: Absolutely. Developing CLIA kits for novel biomarkers is a core strength of our service. Our process starts with extensive feasibility studies and biomarker characterization. We then proceed with custom antibody development or sourcing, followed by iterative assay design and optimization, ensuring that even novel targets can be reliably and sensitively detected.
-
Q: How do you guarantee the reproducibility and accuracy of its CLIA kits?
A: Ensuring reproducibility and accuracy is central to our validation process. We perform extensive studies, including inter- and intra-assay variation, linearity, spike and recovery, and interference testing. Our robust quality control guidelines, coupled with multiple rounds of optimization and rigorous statistical analysis, guarantee the consistent and reliable performance of every kit.
-
Q: What type of post-development assistance do you offer?
A: Our partnership extends beyond the delivery of the developed kit. We offer post-development support, including troubleshooting assistance, technical consultation, and re-validation services for any future modifications or expansions. We are dedicated to ensuring the long-term success and peak functioning of your CLIA kit in the field.
-
Q: Can Creative Biolabs scale up production for commercialization once the kit is developed?
A: Yes, our service includes scalable manufacturing capabilities. Once the CLIA kit development and validation phases are complete, we can transition seamlessly into larger-scale production to meet your commercial demands. Our manufacturing procedures follow strict quality control requirements, assuring consistent performance across all batches.
To assist customers for specific detection of disease-related proteins, Creative Biolabs provides a variety of ELISA based assay kits. If you are interested in our chemiluminescent immunoassay based kits development, please feel free to contact us for more details.
References
- Yasuda, Hiroshi, et al. "Unique biological activity and potential role of monomeric Laminin-γ2 as a novel biomarker for hepatocellular carcinoma: a review." International Journal of Molecular Sciences 20.1 (2019): 226. Distributed under Open Access license CC BY 4.0, the image was modified by extracting and using only Part A of the original image.
- Ma, Ting, et al. "Preparation of an acridinium ester-labeled antibody and its application in goldmag nanoparticle-based, ultrasensitive chemiluminescence immunoassay for the detection of human epididymis protein 4." Micromachines 8.5 (2017): 149.
- Zhu, Jianyu, et al. "An automated and highly sensitive chemiluminescence immunoassay for diagnosing mushroom poisoning." Frontiers in Chemistry 9 (2021): 813219.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.