With abundant experience in the IVD (in vitro diagnostic) antibody development, Creative Biolabs is able to provide a full range of biomarker-specific antibody development services for cancer screening and therapeutic monitoring. Especially, we target different markers of myeloma. Our professional team is optimized to help you with high-quality and cost-effective service to make your project a success.
Aspartate beta-hydroxylase (ASPH)
ASPH is a ~86 kD type Ⅱ transmembrane protein that belongs to the aketoglutarate-dependent dioxygenase family. It catalyzes the hydroxylation of aspartyl and asparaginyl residues in the epidermal growth factor-like repeats in a broad range of proteins, e.g. Notch receptors and ligands. This protein is reported to play an important role in calcium homeostasis. It is expressed from two promoters and undergoes alternative splicing. Among all the isoforms, the longest one (a and f) include a C-terminal Aspartyl/Asparaginyl beta-hydroxylase domain. This domain hydroxylates aspartic acid or asparagine residues in the epidermal growth factor (EGF)-like domains of some proteins. Those proteins include protein C, coagulation factors VII, IX, and X, and the complement factors C1R and C1S. Other isoforms are different primarily in the C-terminal sequence. They are in lack of the hydroxylase domain, and some have been localized to the endoplasmic and sarcoplasmic reticulum. Some of these isoforms are reported to be in complexes with calsequestrin, triadin, and the ryanodine receptor, and have been shown to regulate calcium release from the sarcoplasmic reticulum.
ASPH Marker of Gliomas
Glioblastoma Multiforme (GBM) is the most common malignant primary brain tumor. Despite advances in chemotherapy, neurosurgery, and radiation, median survival of GBM remains between 12 and 15 months following diagnosis. Several key pathophysiological processes, particularly several key factors, are associated with driving the invasive growth of GBM. ASPH is one of the key markers that have been implicated in the cross-talk among all those signaling processes.
ASPH is expressed at high levels in many malignant neoplasms of different histogeneses, and at very low levels or not at all in most normal cells and tissues, including the brain. ASPH's aggressive pro-tumor effects are mediated by gene over-expression, and/or high levels of its protein with attendant increased catalytic activity. Besides ASPH, Humbug, one of its isoforms that lacks a catalytic domain and has a probable role in cell adhesion/calcium flux, is also over-expressed in malignant neoplasms. Like ASPH, high levels of Humbug correlate with aggressive tumor cell behavior and worsened clinical prognosis.
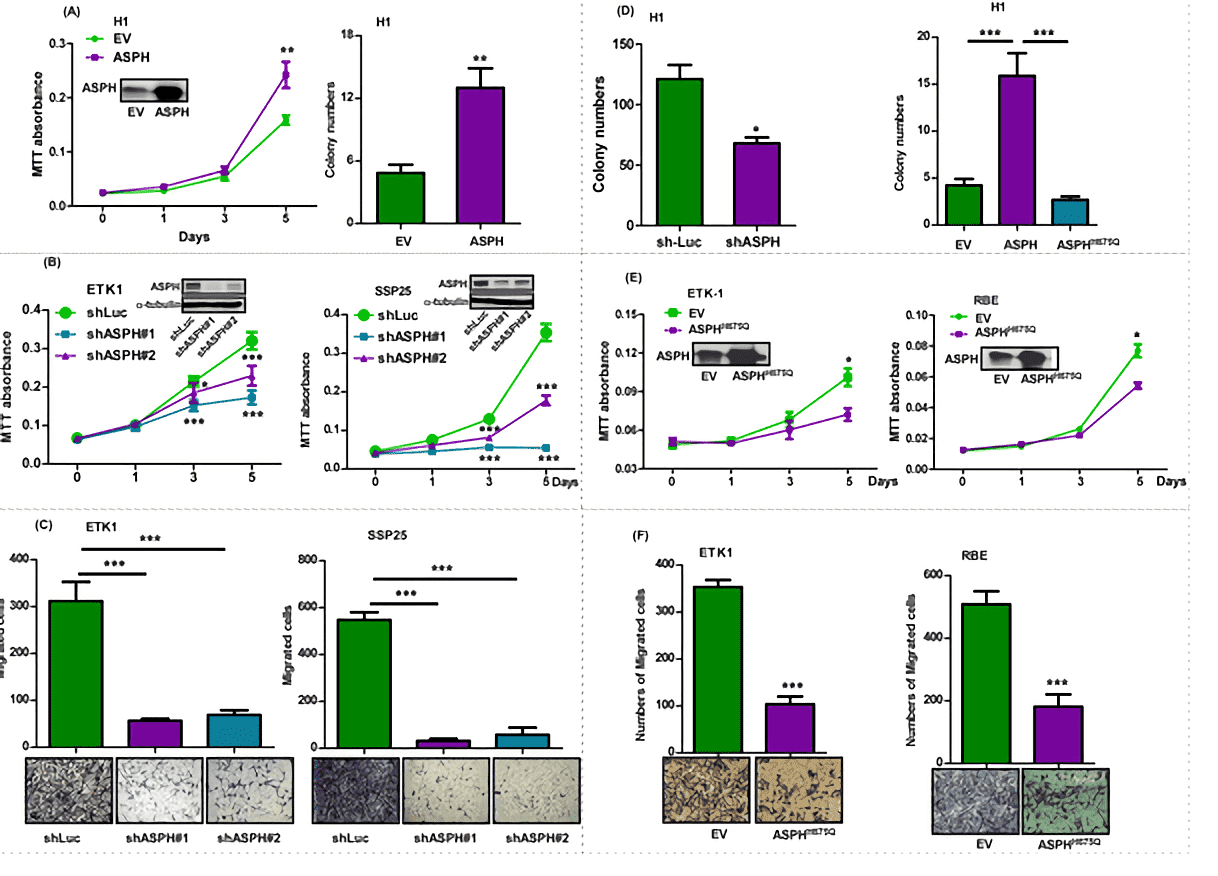 Fig.1 ASPH expression promoted malignant phenotypes in CCA cell lines.1
Fig.1 ASPH expression promoted malignant phenotypes in CCA cell lines.1
IVD Antibody Development Service Targeting ASPH Marker
IVD antibodies are extensively used for disease screening, diagnosis, and therapeutic monitoring. Through our role as a leading antibody service provider, Creative Biolabs is well-positioned to help develop high-quality antibodies targeting ASPH marker of myeloma. Moreover, we offer antibody conjugation, antibody pairing, IVD immunoassay development services to global clients.
For more detailed information, please feel free to contact us or directly sent us an inquiry.
Reference
- Huang, Chiung-Kuei, et al. "Anti-tumor effects of second generation β-hydroxylase inhibitors on cholangiocarcinoma development and progression." PLoS One 11.3 (2016): e0150336. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.