Creative Biolabs is known as a professional antibody service provider. We custom design in vitro diagnostic (IVD) antibody development services targeting different diagnostic markers of multiple diseases. Especially, we focus on the carcinoembryonic antigen (CEA) marker of colorectal cancer.
Carcinoembryonic Antigen
Carcinoembryonic antigen (CEA) was initially discovered an oncofetal antigen expressed in human embryonic colon tissues and in colonic carcinomas in 1965. It is a substance observed on the surface of some cells and is a large glycoprotein (180 kDa molecular weight) encoded by a gene named CEACAM5 which belongs to a subfamily of the immunoglobulin gene superfamily (CEA-related cell adhesion molecule family), located on chromosome 19q13.2. This glycoprotein is produced by the gastrointestinal tract cells during fetal development and expressed in very small amounts after birth. The expression levels of CEA in the bloodstream are thus relatively low unless certain diseases are presented, as well as certain forms of cancer. The report shows that CEA affects carcinogenesis by inducing tumor angiogenesis and by enhancing tumor cell survival. That means CEA has potential to be used as a tumor marker in clinical detections and monitoring.
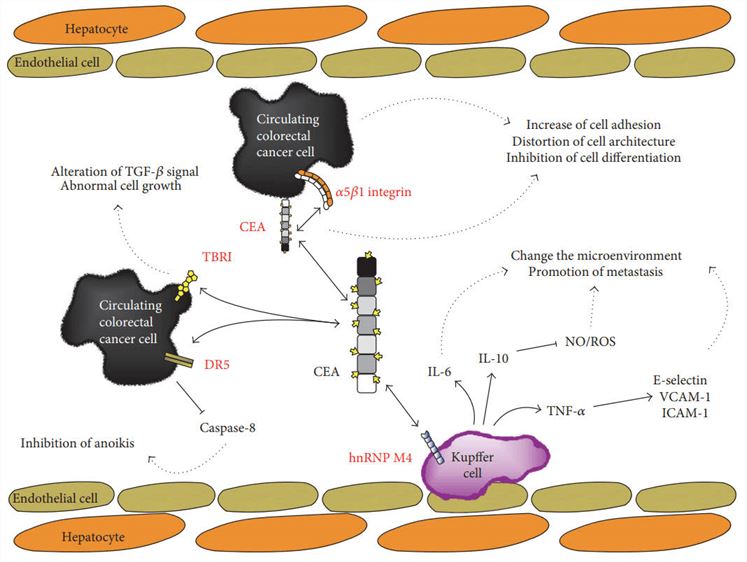 Fig.1 CEA-affecting biological events.1
Fig.1 CEA-affecting biological events.1
CEA and Colorectal Cancer
CEA appears to regulate various cellular functions, such as inhibition of cell differentiation, polarization, intercellular adhesions, signal transduction, cellular migration, anoikis and tissue architecture. In normal colons, CEA is released into the lumen from the apical surface and then excreted into feces. However, CEA in colorectal cancer is expressed throughout the entire cytoplasmic membrane and is delivered into the vascular or lymphatic spaces via intercellular spaces. Its levels have been increased in approximately 60%-85% of patients with colorectal carcinoma (CRC). CEA is a high molecular-weight protein and functions as an intercellular adhesion molecule promoting the aggregation of human CRC. As tumors grow, the concentration of CEA in the blood generally goes rising, though some tumors do not generate CEA.
CEA is one of the most widely accepted tumor markers and is associated with enhanced metastatic potential in CRC. Through radioimmunoassay tests of serum in 1969, it was identified to be a glycosylphosphatidylinositol cell surface-anchored glycoprotein that serves as a functional CRC ligand of the cell adhesion molecules L-selectin and E-selectin, which is important for cancer cell migration. Importantly, it is produced on the luminal surface of the colonocytes and its expression pattern changes in the neoplastic cell so that it is also expressed on the basal and lateral cell membranes. Now, it is acknowledged to play a critical role in establishing and maintaining cellular architecture and function in the colon.
Carcinoembryonic Marker for CRC
CEA has specificity for CRC of 90%, but a sensitivity of only 40%-75%, hence t so it is of more application for monitoring than for screening or diagnosis. Routine monitoring of CEA levels is standard operation in patients with resected colorectal tumors. This biomarker has been investigated extensively for disease recurrence in patients undergoing curative intent-resection of a primary CRC. Patients with resected colorectal tumor should normalize their CEA expressions within weeks from surgery, and failure to do so by four months which is highly suspicious for some systemic disease.
CRC is a heterogeneous disease with long-term outcomes and responses to treatment. Recent advances in the genetic and molecular features of carcinomas have yielded a set of predictive markers that assist the identification of patients at higher risk for disease recurrence and progression. At Creative Biolabs, we offer high-quality antibody (pair) development services to global clients by taking advantage of our advanced technologies. Moreover, we offer IVD immunoassay development services of various formats, including ELISA, lateral flow assay, chemiluminescence assays, turbidimetric assays, etc. Bringing years of experience and expertise, our dedicated team of professionals will help you with every step of the development process to bring your immunoassay to market rapidly. Contact us to discuss your project and experience the great value of our services.
Reference
- Lee, Joo Han, and Seong-Wook Lee. "The roles of carcinoembryonic antigen in liver metastasis and therapeutic approaches." Gastroenterology research and practice 2017 (2017). Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.