In vitro diagnostic (IVD) antibodies are essential elements in immunodiagnostic kits that are used for disease diagnosis and prognosis. As a well-known antibody service provider, Creative Biolabs devotes to launching customized IVD antibody development services for diverse human diseases and a broad range of diagnostic markers. Here, we focus on the cytokeratin 20 (CK20) as a marker of colorectal cancer.
Cytokeratin and CK20
Cytokeratin (CK) belongs to one of the intermediate filaments and is subdivided into cytokeratins and hair keratin. It is a member of among approximately twenty cytoskeletal structural proteins present in normal epithelial cells and responsible for the structural integrity of epithelium. The expression of CK is maintained during the malignant transformation, which is a powerfully useful tool in oncology diagnostics. Since CK expression is often preserved in tumorous cells, the application of specific antibodies is valuable in determining the origin of metastatic carcinomas.
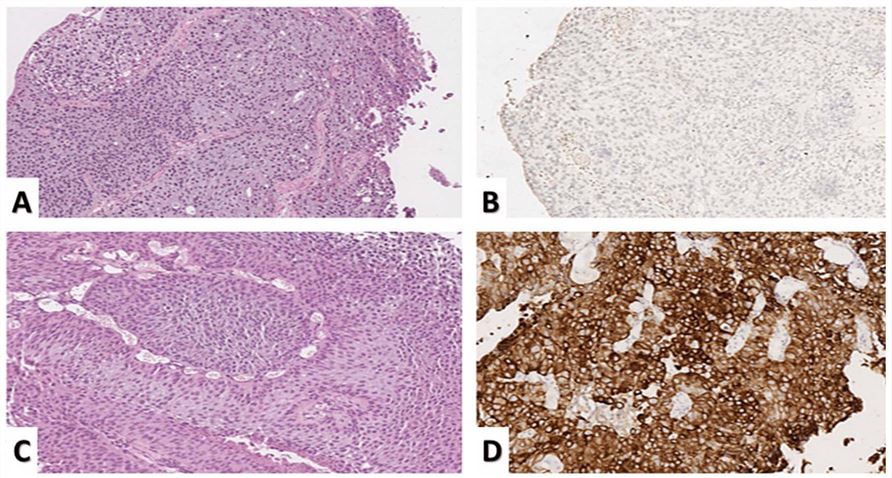 Fig.1 CK20 expression in urothelial carcinoma.1
Fig.1 CK20 expression in urothelial carcinoma.1
CK20 (Cytokeratin 20) is an intermediate filament protein with 46 kDa. It is a low molecular weight member of the CK protein family, which is restricted to express in gastric and intestinal epithelial cells, urothelium, and Merkel cells, as well as in primary colorectal carcinomas and their metastases. Essentially, CK20 is non-reactive in squamous cell cancers, adenocarcinomas of lungs, breasts, and endometria, and non-mucinous tumors of ovaries and small cell carcinomas. CK20 has been identified as a unique type I cytokeratin. The type I keratin consists of acidic proteins arranged in pairs of heterotypic keratin chains and its gene is clustered in a region of chromosome 17q12-q21. CK-based tumor biomarker tests may be recognized as simple, cheap, noninvasive, and reliable predictive methods and offer a sight for more efficient management compared to conventional approaches.
CK20 and CRC
Colorectal carcinoma (CRC) remains one of the major cancer-related deaths despite some progress of treatments gained last years. Detection of this disease at earlier stages can reduce its mortality to large extent. Higher expression level of CK20 in patients maybe is regarded as a promising sign for the diagnosis of diseases in non-metastatic stages. Current data suggest that the identification of CK20 as relative sensitive markers has potential to be of value choice for primary diagnosis of CRC in early phases.
The correlations between the expression level of CK20 and efficacy of CRC treatment and postoperative prognosis are evaluated to demonstrate the clinical value of CK20. Postoperative follow-up is performed on 62 patients who have undergone surgery for CRC in two years. The collected specimens are tumor tissues and intraperitoneal drainage fluids of these patients, together with blood samples during the two-year follow-up period. The relationship between the CK20 levels and postoperative outcomes is analyzed by Spearman correlation analysis. Both in tumor samples and intraperitoneal drainage fluids, CK20 levels are lower in patients with earlier stages than in those at later stages. During postoperative follow-up, patients with serum negative CK20 have significantly higher three-year survival rates than serum positive CK20 patients. CK20 expressions can provide valuable clinic information on the postoperative prognosis of CRC patients.
IVD Antibody Development Service Targeting CK20 Marker
CRC is one of the most common malignant tumors with a three-year survival rate of approximately 70% after surgical resection. There still is a trend to increase mortality, though modern cancer therapeutics can manage and control the progression of CRC effectively. Currently, techniques for the CRC evaluation include observation of clinical symptoms, pathological examination and tumor marker level measurement. However, they are all appearing inherent limitations that lead to challenging in detecting early stage carcinomas and lymph node metastases.
The considerable progress has been achieved towards improving survival of patients. Because more than 60% of CRC is identified at the symptomatic phases with a lower rate of long-term survival, so thus, it is critical to diagnose the cancer indication at the earlier asymptomatic stages with effective screening. By evaluating mRNA expression of specific tumor markers, cancer cells can be detected in peripheral blood of cancer patients compared with healthy controls. The reason is that the tumor cells shed from the primary carcinoma mass into the bloodstream can be tracked in the early phases of the disease. Lots of tumor markers, such as CK20, have been revealed to be stable and specifically expressed in primary and metastatic CRC and have been picked for the efficient detection of circulating cancer cells in peripheral blood.
With our versatile IVD platform, Creative Biolabs is proud to develop novel CK20-specific antibody from scratch to commercial IVD kit (we can also start with provided antibody candidates). Besides, we help develop high-quality IVD immunoassays of different formats, giving expert support in feasibility analysis, protocol establishment, assay design, validation, and kit production. Our services are customized to suit the specific requirements of our clients. Please contact us for more details if you are interested in our service.
Reference
- Popov, Hristo, George S. Stoyanov, and Peter Ghenev. "Role of Cytokeratin 20 as a Predictive and Prognostic Marker in Urothelial Neoplasms." Cureus 14.11 (2022). Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.