Renal cell carcinoma (RCC) accounts for 2% to 3% of malignancies in adults and causes more than 100,000 deaths per year. Genetic aberrations in RCC commonly include loss of function of the VHL gene in clear cell RCC, overexpression of the c-MET gene in papillary RCC type I, deficiency in the FH gene in papillary RCC type II and loss of heterozygosity of the BHD gene in chromophobe RCC. It is necessary to deeply understand the mechanisms of RCC for promoting treatment outcomes.
Epigenetics refers to functionally relevant alternations in the genome that affect the expression of specific genes without the involvement of DNA damage. The common epigenetic changes comprise DNA methylation, aberrations in non-coding RNAs and posttranslational modifications of histone. Histone methylation, also a type of histone modifications, represents a process by which methyl groups are transferred to lysine (K) or arginine (R) residues. Methylation of histone influences lots of biological processes in the context of cellular responses, abnormality of which leads to various disorders including cancer. There is cumulative evidence that histone demethylases (HDMs), key players in histone methylation, play important roles in renal cell cancers. Creative Biolabs has always been an experienced specialist in the antibody production field. Now we offer high-quality IVD antibody development services to support the development of IVD kits for renal cancer diagnosis.
Epigenetics and Cancer
Altered epigenetic homeostasis is implicated in tumorigenesis and epigenetic-based biomarkers, which may assist in diagnosis, prognosis, as well as prediction of response to targeted therapy. In particular, histone modification and chromatin modulator have been acknowledged to play a central role in cancer progression. In RCC, certain histone modifications correlate with progression-free survival and pathological characteristics of tumors. Additionally, defects in epigenetic enzymes, involved in chromatin remodeling and packaging, have participated in the development of RCC, reflecting the role of these mechanisms in renal oncogenesis. Herein, increasing focus aimed to investigate the potential of HDMs expression as biomarkers of metastatic progression in renal cancer.
The first histone demethylase KDM1 was discovered in 2004 and at that time histone methylation was widely thought to be irreversible. Two years later, several jumonji C (JmjC)-domain-containing demethylases were observed and subsequent studies testified that histone methylation is reversible. HDMs work as key factors in eukaryotic transcription, no matter activation or repression, and other chromatin-dependent processes including DNA damage and chromosome condensation. These HDMs controls gene expression and influences the cell fate, most of whom are associated with human diseases, such as RCC.
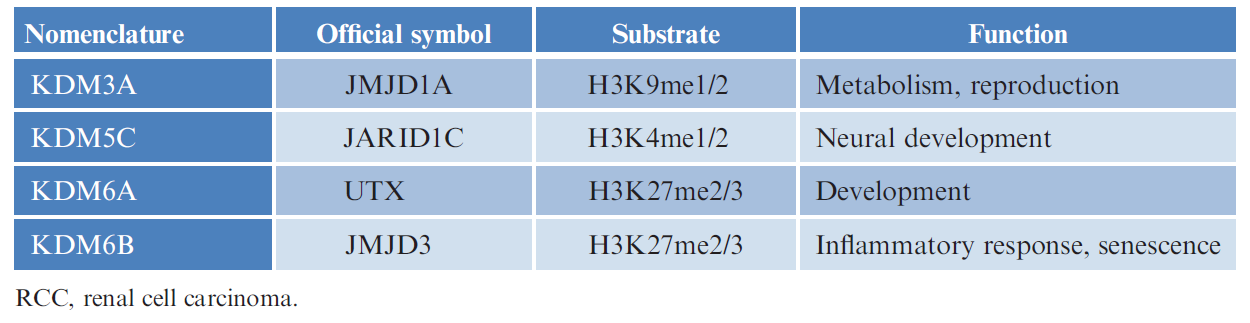 Fig.1 Histone demethylases implicated in RCC. (Guo, X., 2017)
Fig.1 Histone demethylases implicated in RCC. (Guo, X., 2017)
HDMs and RCC
- KDM3A (JMJD1A, JHDM2A)
KDM3A is an H3K9me1/2 demethylase of JmjC family. The research has reported that overexpression of KDM3A is linked to RCC development. Renal cancer specimens from patients expressed a higher expression of KDM3A when compared to normal non-cancerous tissues of the kidneys. And KDM3A levels are expressed highly around blood vessels in RCC samples.
- KDM5C (JARID1C)
KDM5C is an H3K4me1/2 demethylase, whose abnormality also has relation to cancer development. The assay that inactivating mutations of KDM5C are identified in 101 clear cell RCC cases made use of massively parallel sequencing technologies. Further studies in 132 patients with clear cell RCC demonstrated that KDM5C is mutated in 4% of the cases.
- KDM6A (UTX) and KDM6B (JMJD3)
Both KDM6A and KDM6B are a type of H3K27me2/3 demethylase. KDM6A is essential for normal fetal development and tissue-specific differentiation. In renal cancer cells, inactivating somatic mutations of KDM6A have been confirmed. There are results showed that KDM6A levels are upregulated in RCC. When it comes to KDM6B, it is also over-expressed in renal cancers and may be involved in oncogene-induced senescence. They are important regulatory factors that modulate gene expression and appear proto-oncogene potential in RCC.
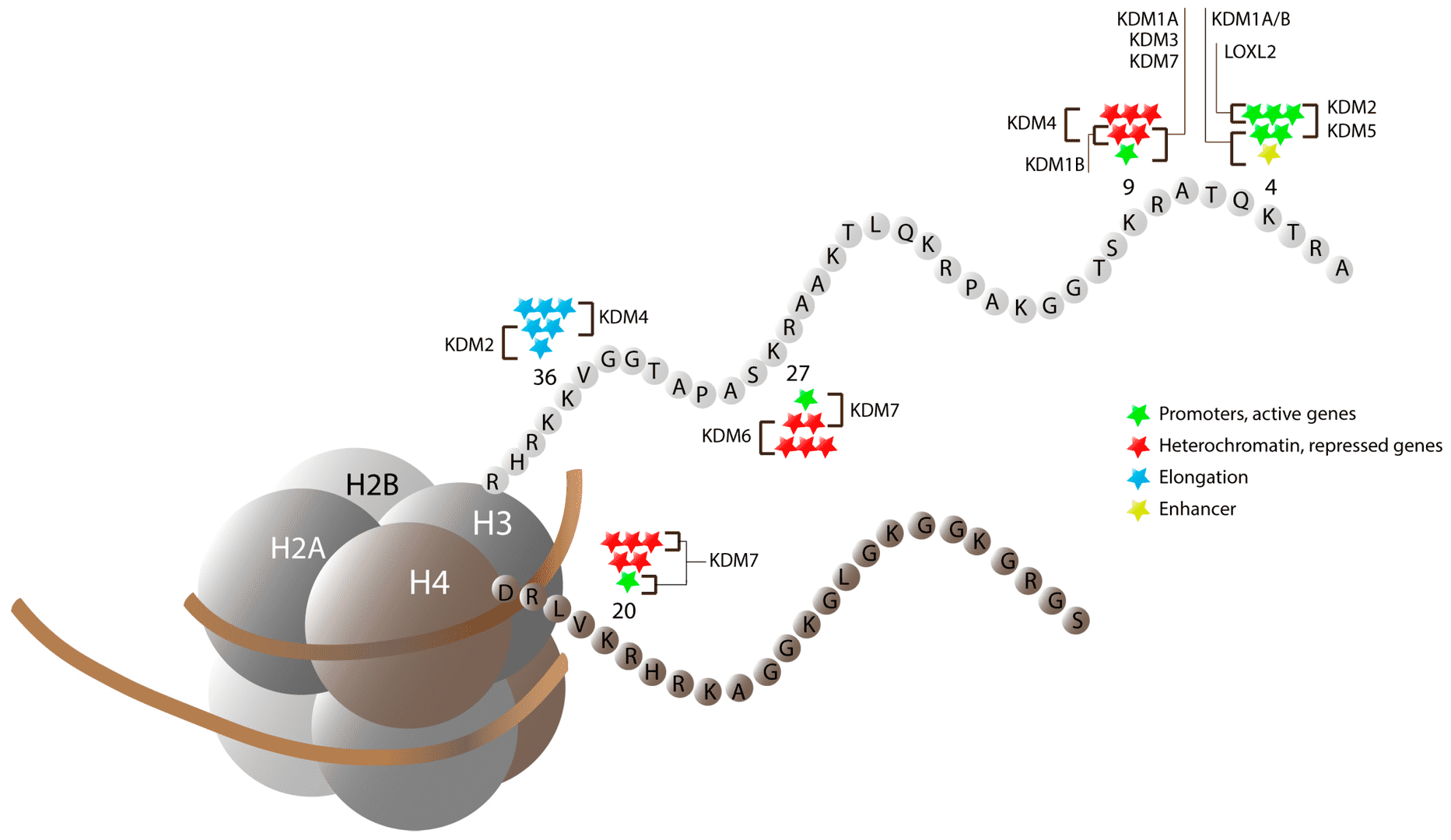 Fig.2 Lysine-specific histone methylation and the activity of histone demethylases. (Verde, G., 2017)
Fig.2 Lysine-specific histone methylation and the activity of histone demethylases. (Verde, G., 2017)
IVD Antibody Development Service for HDM Marker
With extensive experience and advanced technologies, Creative Biolabs has been recognized as an expert in the field of IVD antibody development. Our scientific team is able to provide polyclonal, monoclonal, and recombinant antibody development services for diagnostic use. Besides, we help develop high-quality IVD immunoassays of different formats, giving expert support in feasibility analysis, protocol establishment, assay design, validation, and kit production. Our services are customized to suit the specific requirements of our clients.
If you are interested in the services we provide, please feel free to contact us or directly send us an inquiry.
References
- Guo, X., (2017). “The Emerging Role of Histone Demethylases in Renal Cell Carcinoma.” J Kidney Cancer VHL, 4(2), 1-5.
- Verde, G., (2017). “Lysine-Specific Histone Demethylases Contribute to Cellular Differentiation and Carcinogenesis.” Epigenomes, 1(1), 4.
For Research Use Only.