In vitro diagnostic (IVD) tools are increasingly attracting more attention from all around the world because of their importance in disease prognosis and diagnosis, Creative Biolabs, as an experienced expert in the antibody production field, now offers high-quality IVD antibody development services to support the development of IVD kits for renal cancer diagnosis.
It is well-known that renal cancer is the most lethal of the common urological malignancies. Renal cell carcinomas (RCC), originating from renal tubule cells, is the most frequent kidney tumors and accounts for 85%-90% of all cases. RCC comprises several different histological subtypes, each with distinct morphologic, genetic, and clinical features. Besides, more than 50 histone methyltransferases (HMTs) have been identified thus far, and unlike other histone modifications, methylation does not influence the charged state of the residues. Thus, the effect on gene expression depends on the residue and methylation level (mono-, di-, or tri-methylation). In RCC, the deregulation of chromatin machinery has been increasingly regarded as a significant mechanism of neoplastic transformation, highlighting its potential character as a diagnostic and prognostic biomarker.
Histone Methylation and Disease
Many studies have indicated that epigenetic modification plays a crucial role in cancer development. Histone methylation is essentially a post-translational and epigenetic modification of chromatin that determines gene expression, genomic stability, genetic imprinting, DNA methylation, cell mitosis and lineage development. Histone methylation occurs at certain amino acids containing lysine (K) and arginine (R) in the N-terminus of histones H3 and H4, by the addition of one, two, or three methyl groups. And here, two major types of HMTs exist, lysine-specific and arginine-specific, for whom S-Adenosyl methionine (SAM) serves as a cofactor and methyl donor group. In eukaryotic cells, HMTs overcome the inaccessibility by modifying histones at certain sites through methylation that the genome is tightly condensed into chromatin.
Deregulation of some HMTs has also been linked to cancer aggressiveness. The prevailing opinion is that cancer evolution is driven by the sequential acquisition of both genomic and epigenomic alterations, consequently causing the activation of oncogenes as well as the functional loss of tumor-suppressor genes. Remarkably, in contrast to genetic lesions, epigenetic abnormalities are potentially reversible, accepting for therapeutic intervention.
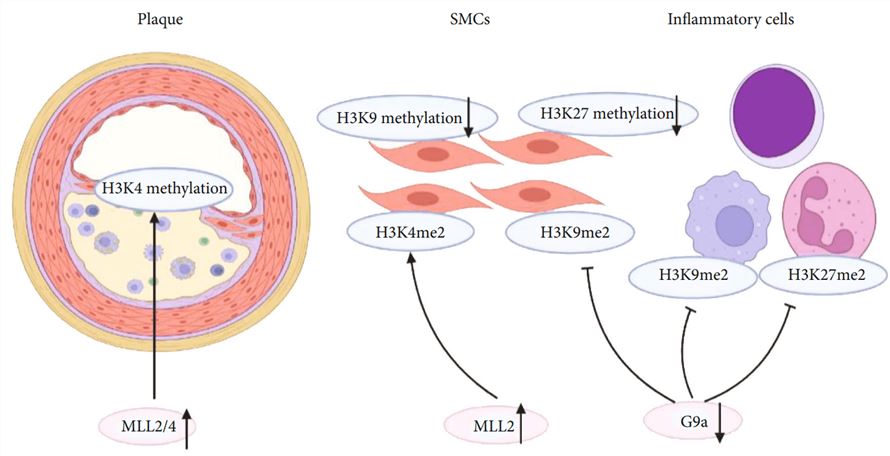 Fig.1 Histone methylation in the initiation and progression of atherosclerosis.1
Fig.1 Histone methylation in the initiation and progression of atherosclerosis.1
HMTs and RCC
RCC is generally resistant to chemotherapy and radiation therapy and will recur even after nephrectomy with curative intent. Besides, there is lack of specific diagnostic markers for RCC, which is an important contribution to its poor prognosis. Epigenetic events, comprising aberrations in DNA methylation patterns, non-coding RNAs expression, and deregulated chromatin machinery, play a critical part in neoplastic transformation, involving renal carcinogenesis. Histone methylations are important biologically since it is a principal epigenetic modification of chromatin. There are studies found that SETDB2 and RIOX2, encoding for an HMT and an HDM respectively, expression levels are significantly altered in renal normal tissues (RNTs). These two genes are further selected for validation by quantitative RT-PCR in 160 renal cell tumors (RCTs). After a series of assays, the final results conclude that SETDB2 and RIOX2 might be involved in renal tumorigenesis and RCC progression, especially in metastatic spread.
The field of epigenetic therapy is expanding rapidly and inhibitors targeting DNA methyltransferases and histone deacetylases have been approved for treating certain blood cancers and are being tested for other cancers in clinical trials. Currently, Creative Biolabs is focusing on the promising role of HMTs in renal cancers and makes surely progress in IVD antibodies for RCC. With the assistance of us, there will be a better understanding of the molecular basis of renal cancer that is able to facilitate the purpose of developing effective and more selective diagnostic and therapeutic approaches.
Reference
- Yi, Xin, et al. "Histone methylation and oxidative stress in cardiovascular diseases." Oxidative Medicine and Cellular Longevity 2022 (2022). Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.